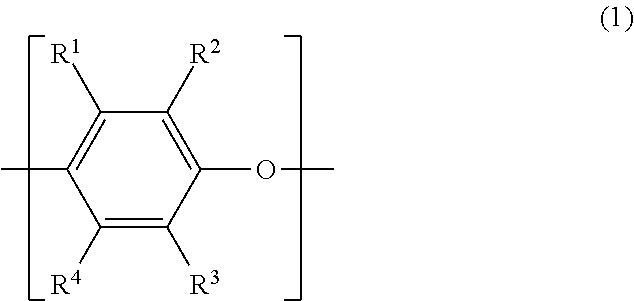
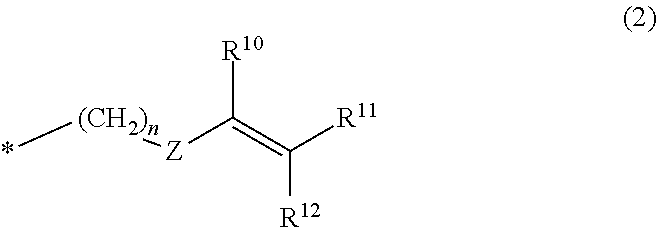
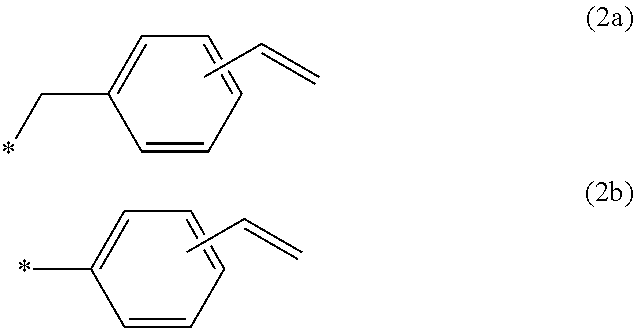
Polyphenylene ether resin composition, prepreg, metal-clad laminate
a technology of polyphenylene ether and composition, which is applied in the direction of metal layered products, electrical appliances, printed circuits, etc., can solve the problems that metal-clad laminate plates produced using conventionally known polyphenylene ether resin compositions sometimes have insufficient and achieve excellent heat resistance and water resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
production example 1
[0056]Into a 500 mL flask, 151.95 g of tetrahydrofuran (hereinafter, abbreviated as THF) and 19.65 g of hexane were added. After the mixture was cooled to −40° C., 2.28 g of n-butyllithium (a hexane solution with a concentration of 15.1% by weight) was added and stirred for 10 minutes, then 11.99 g of styrene was added dropwise, and the reaction was continued for 30 minutes. The solution was measured by gas chromatography (hereinafter, abbreviated as GC) and the disappearance of monomers was confirmed. Then, a mixed solution of 21.44 g of 1,3-butadiene, 23.43 g of THF, and 7.80 g of hexane was added dropwise, and the reaction was continued. After the solution was measured by GC and the disappearance of monomers was confirmed, 12.05 g of styrene was added dropwise, and after 30 minutes, 0.51 g of methanol was added to terminate the reaction.
[0057]The copolymer obtained was analyzed by gel permeation chromatography (mobile phase: THF, polystyrene standards), and it was confirmed that ...
production example 2
[0059]Into a 500 mL flask, 149.37 g of THF and 17.53 g of hexane were added. After the mixture was cooled to −40° C., 5.21 g of n-butyllithium (a hexane solution with a concentration of 15.1% by weight) was added and stirred for 10 minutes, then 10.47 g of styrene was added dropwise, and the reaction was continued for 30 minutes. The solution was measured by gas chromatography (hereinafter, abbreviated as GC) and the disappearance of monomers was confirmed. Then, a mixed solution of 49.28 g of 1,3-butadiene and 49.28 g of THF was added dropwise and the reaction was continued. After the solution was measured by GC and the disappearance of monomers was confirmed, 10.66 g of styrene was added dropwise, and after 30 minutes, 1.12 g of methanol was added to terminate the reaction.
[0060]The copolymer obtained was analyzed by gel permeation chromatography (mobile phase: THF, polystyrene standards), and it was confirmed that the molecular weight (Mw) was 14,200 and the molecular weight dist...
production example 3
[0062]Into a 500 mL flask, 155.90 g of cyclohexane and 20.10 g of THF were added. The mixture was warmed to 30° C., 1.95 g of n-butyllithium (a hexane solution with a concentration of 15.1% by weight) was added and stirred for 10 minutes, then 7.64 g of styrene was added dropwise, and the reaction was continued for 30 minutes. The solution was measured by gas chromatography (hereinafter, abbreviated as GC) and the disappearance of monomers was confirmed. Then, a mixed solution of 35.07 g of 1,3-butadiene and 35.07 g of cyclohexane was added dropwise and the reaction was continued. After the solution was measured by GC and the disappearance of monomers was confirmed, 7.78 g of styrene was added dropwise, and after 30 minutes, 0.40 g of methanol was added to terminate the reaction.
[0063]The copolymer obtained was analyzed by gel permeation chromatography (mobile phase: THF, polystyrene standards), and it was confirmed that the molecular weight (Mw) was 17,400 and the molecular weight ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com