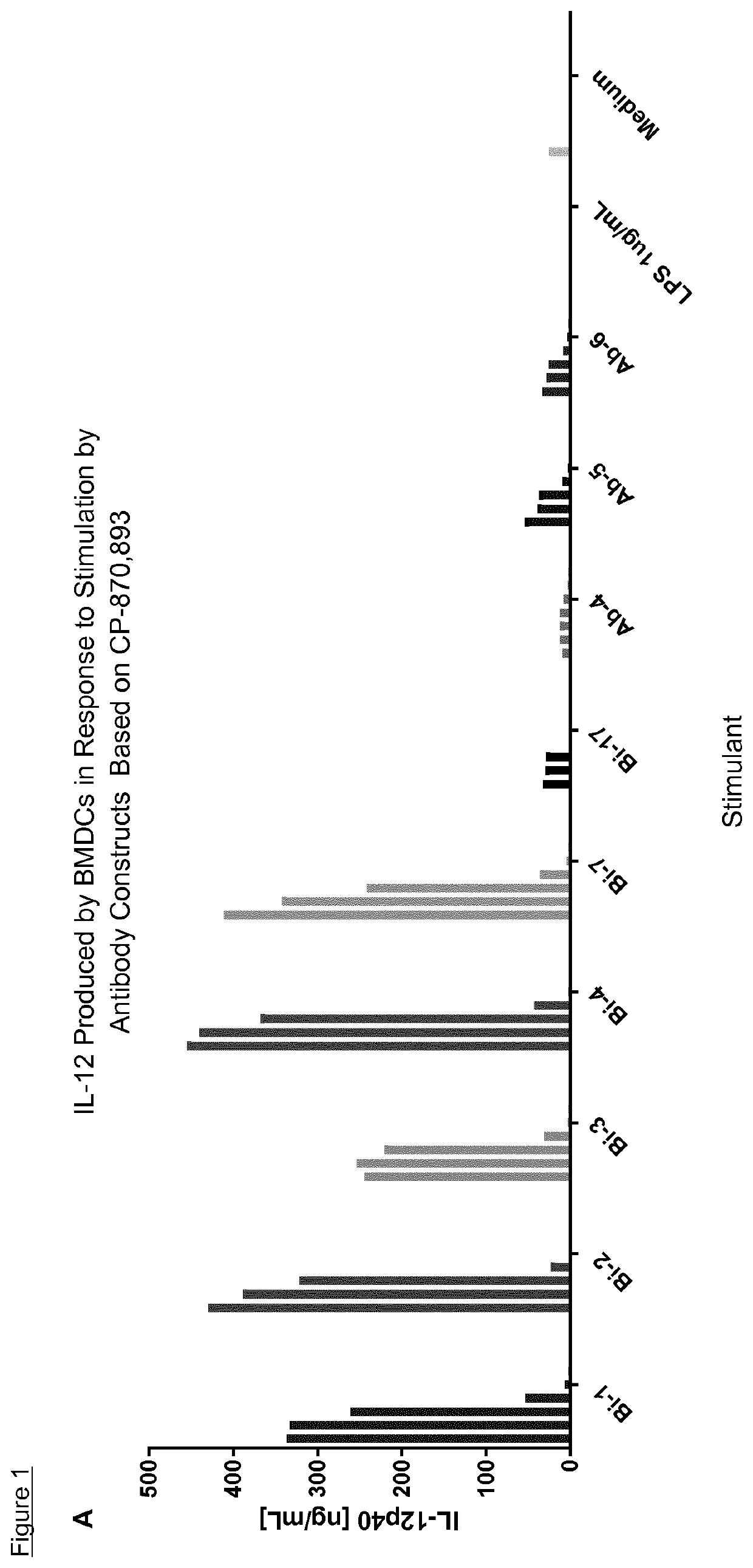
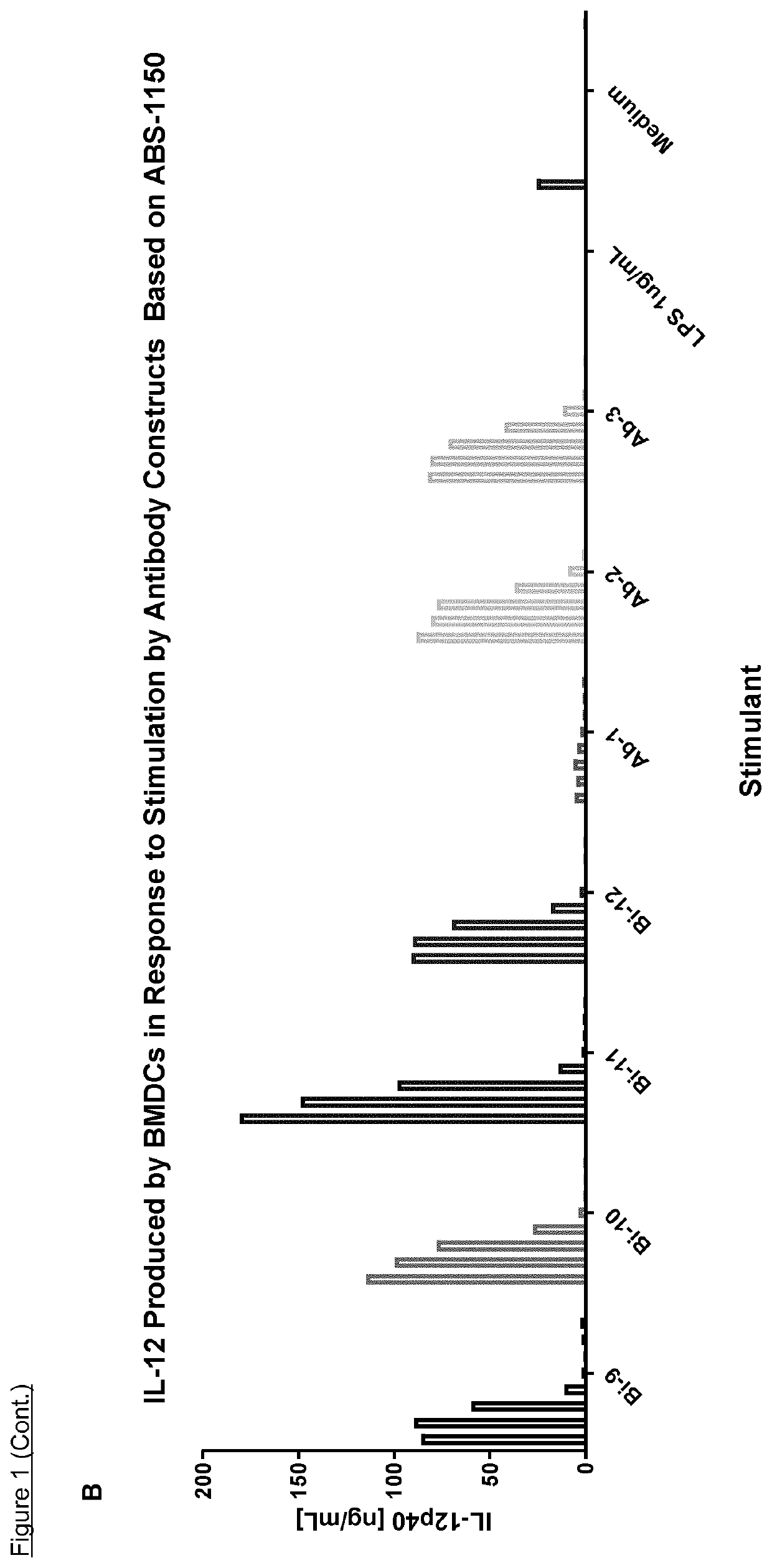
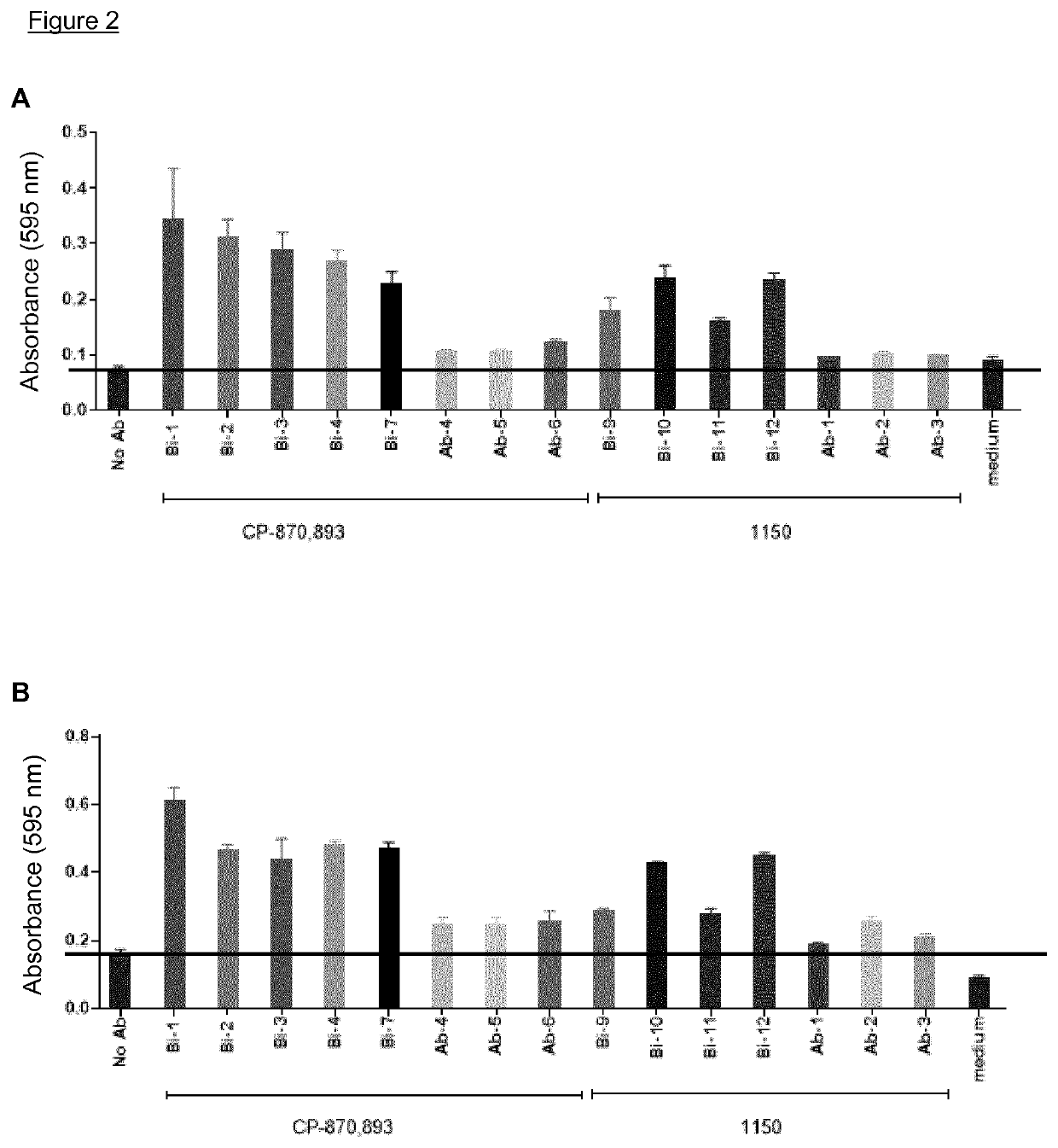
Bi-specific conjugates
a conjugate and bi-specific technology, applied in the field of new drugs, can solve the problems of poor efficacy of cd40 agonists in leading to effective t-cell activation, insufficient cd40 stimulation for t-cell activation, and inability to always be antigenic materials, so as to facilitate the preparation of patient-specific therapeutic agents and improve the efficacy of complexes. effect and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Cells
[0255]B3Z (Karttunen et al., PNAS 89(13): 6020-6024, 1992), a murine T-cell hybridoma expressing a TCR which recognises the ovalbumin peptide OVA257-264 (SIINFEKL, SEQ ID NO: 1) in the context of the murine Class I MHC H-2Kb, was used to assess peptide loading and subsequent antigen presentation. B3Z cells express β-galactosidase under the control of the IL-2 promoter and thus, upon T-cell activation and proliferation, the enzyme will be expressed. β-galactosidase is able to hydrolyse the substrate chlorophenol red-β-D-galactopyranoside (CPRG), which leads to a colour change with a magnitude corresponding to the level of B3Z T-cell activation. Hence B3Z activation may be detected and measured by spectrophotometry.
[0256]Pmel-1 mice (Jackson Laboratory (USA), mouse strain 005023, described further below) are transgenic mice with T-cells which express a TCR specific for the murine gp100(25-33) peptide (SEQ ID NO: 2, amino acids 25-33 of the melanoma antigen gp...
example 2
Spread of T-Cell Activation Following Therapeutic Administration
Materials and Methods
[0289]Adult female C57BL / 6 mice (18-20 g weight) were administered CFSE (carboxyfluorescein succinimidyl ester)-labelled splenocytes from Pmel-1 mice and tghCD40 immature BMDC as described in Example 1 above (see “In Vivo Experimental Setup”). On day 1, various combinations of antibodies and UU-30 peptide were administered as described above. The antibodies used in this example were Ab-2 and Bi-10, which were administered at doses of 7.5 pmol (low, L); 15 pmol (medium, M); and 22.5 pmol (high, H). The UU-30 peptide was administered at doses of 18.75 pmol (L), 37.5 pmol (M) and 56.25 pmol (H).
[0290]After 72 hours, the draining popliteal and non-draining inguinal lymph nodes were harvested and passed through a 70 μm cell strainer to obtain single cell suspensions. Pmel-1 cell accumulation and proliferation was assessed by flow cytometry. Pmel-1 T-cells were gated out based on expression of the congeni...
example 3
of the Interaction Between a Tag and an scFv on a Tetravalent Antibody
Materials and Methods
[0292]The bispecific antibody Bi-17 contains the FITC-8 scFv, which recognises FITC. The scFv is located at the C-terminus of the heavy chain of the anti-CD40 antibody (i.e. it is encoded C-terminal to the IgG2 constant region). The amino acid sequence of the FITC-8 scFv is set forth in SEQ ID NO: 70.
[0293]The Bi-17 bispecific antibody was produced by Absolute Antibody (UK). The antibody was expressed in HEK293 cells, and purified by affinity chromatography using protein A followed by preparative size exclusion chromatography (SEC). The purity was determined by SDS-PAGE to be >98% and monomeric content determined to be 94% by analytical SEC. Endotoxin levels were <1 EU / mg as determined by LAL chromogenic endotoxin assay.
CD14 Monocyte and PBMC Isolation.
[0294]Peripheral blood mononuclear cells (PBMCs) were isolated from Buffy Coats, donated by healthy volunteers, by Ficoll separation using SepM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com