Preeclampsia biomarkers and related systems and methods
a biomarker and preeclampsia technology, applied in the field of preeclampsia biomarkers, can solve the problems of non-ideal identification (or ruling out) of preeclampsia using the classical clinical symptoms of the disease, significant morbidity and mortality risks for both mothers and infants, and billions of dollars in healthcare costs annually
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
reliminary Study Design
[0179]Samples of serum and urine for biomarker analysis were obtained from a Progenity Multicenter specimen procurement study, which was designed to, inter alia, identify biomarkers that improve the detection or ruling out of preeclampsia with improved performance relative to the sFlt / PlGF ratio method described by Zeisler et al. (NEJM 274(2017):13-22). More particularly, pregnant women who were 18 years or older (20 weeks to 39 weeks of gestation at first visit) with suspected preeclampsia (based on new onset symptoms, elevated blood pressure, proteinuria, edema or others) were selected for participation. Baseline procedures were performed, including collection of demographic, medical and obstetric histories, list of concomitant medications, weight, height, blood pressure, and other clinical information, as well as obtaining blood and urine samples for use in biomarker assays. After discharge, all patients in the study were followed by interim research visits...
example 2
ation of Protein Biomarkers
[0187]Serum samples collected according to a standard procedure from 68 patients (one non-preeclampsia and one preeclampsia sample failed quality control checks) were analyzed retrospectively. Briefly, filled red top blood collection tubes were allowed to clot at room temperature for 30-60 minutes, were centrifuged 20 minutes at 1300 g to remove the clot, and were then aliquoted for long term storage below −80° C. Hemolysis, date and time of blood collection, and date and time of freezing were recorded. Both single analyte analysis of suspected biomarkers (of Fibronectin, PlGF, sFlt1, and PAPP-A) and unbiased panel analyses of proteins associated with inflammation, immune response, oncology, organ damage, immunooncology, and metabolism (containing 92 biomarkers in each panel) were performed. PlGF was represented in both single analyte analysis and the unbiased panel analysis, whereas sFlt1, PAPP-A, and Fibronectin were not.
[0188]Single Candidate Analyte An...
example 3
otein Screening for Distinguishing Nonpreeclampsia from Preeclampsia
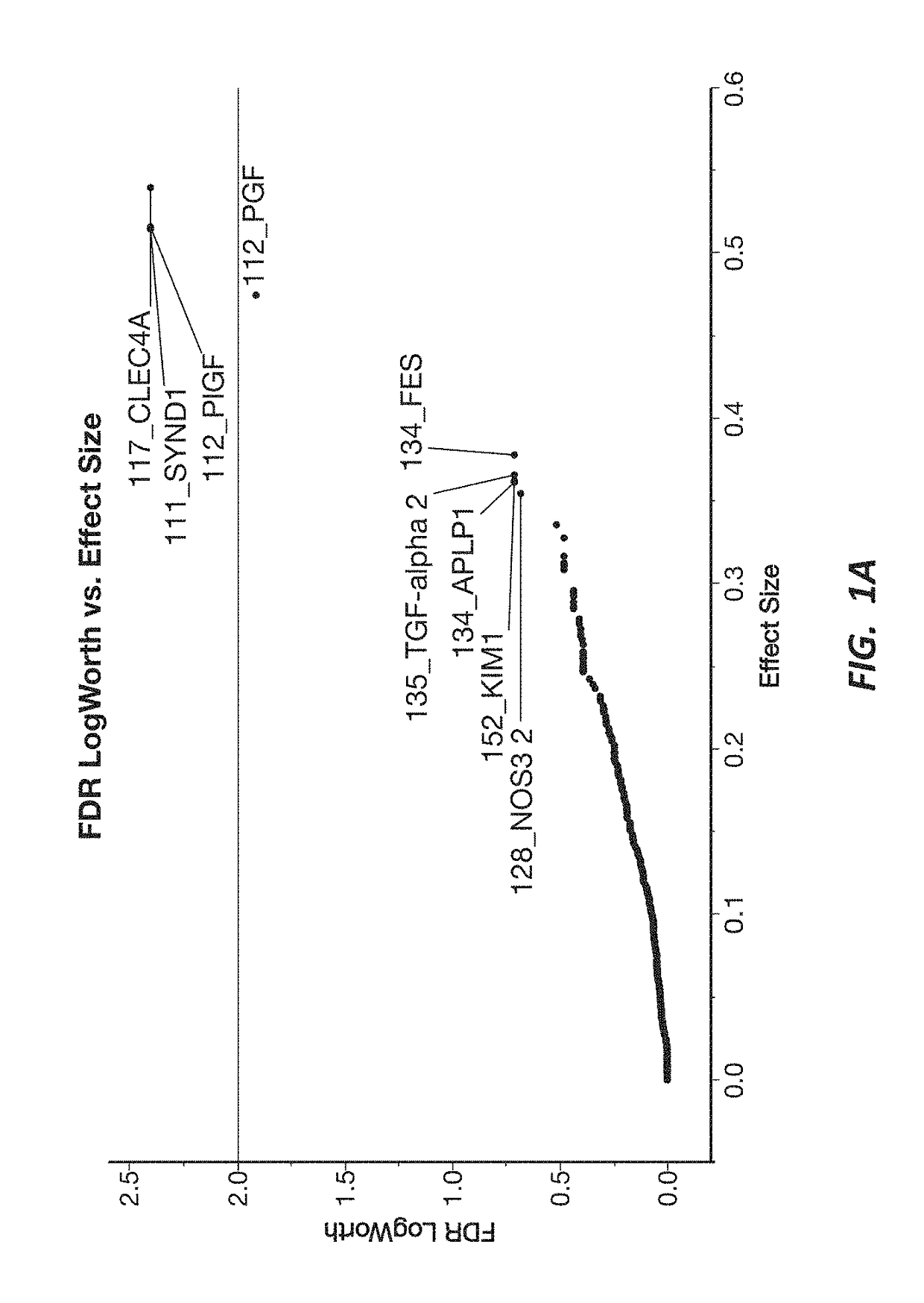
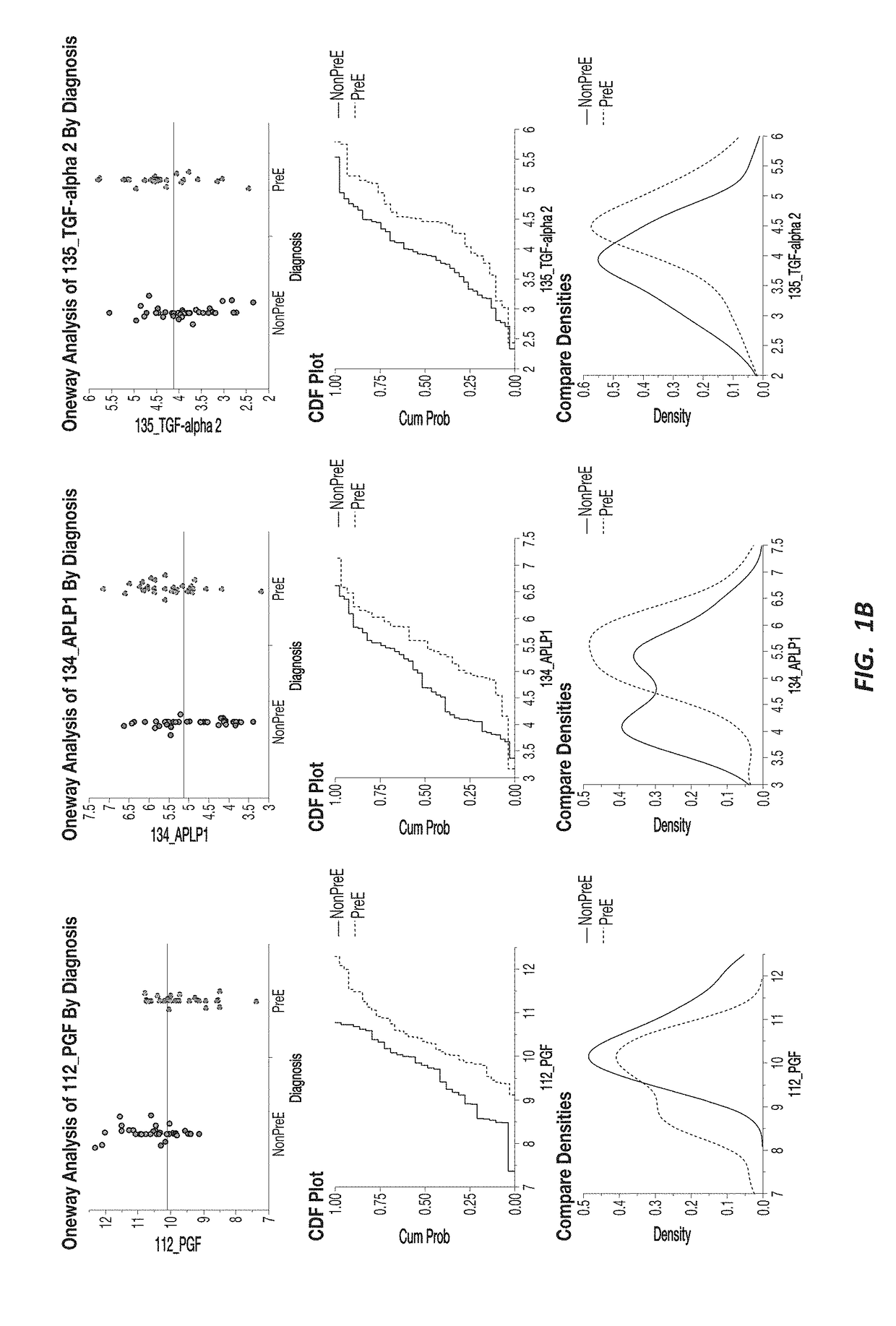
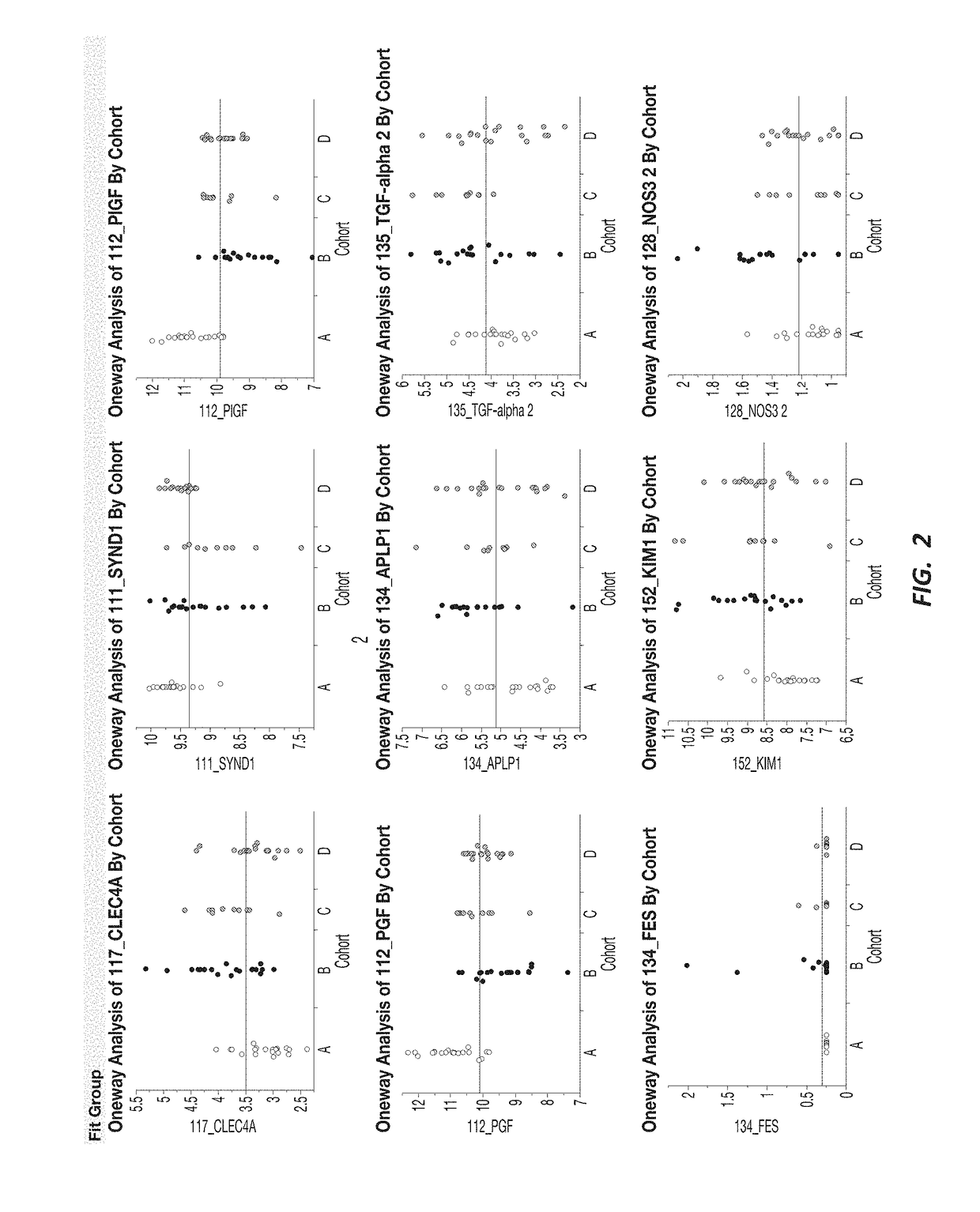
[0192]A response screening of non-preeclampsia vs preeclampsia using ANOVA for each of the 552 markers was performed, defining a FDR LogWorth >2 as significance. A plot of FDR LogWorth vs Effect Size (FIG. 1A) was generated to analyze the value of single biomarkers for distinguishing Nonpreeclampsia vs PreE. Three biomarkers (CLEC4A, SYND1, and PlGF) meet the FDR LogWorth criteria for significance (>2), while 6 additional biomarkers (PGF, FES, TGF-alpha 2, APLP1, KIM1, and NOS32) show an FDR LogWorth more significant than most of the biomarkers. A summary of these top distinguishing markers is provided in Table 2; while visual representations of the data spread for each of the top 3 biomarkers for non-preeclampsia vs preeclampsia is shown in FIG. 1B.
TABLE 2Top Nine Biomarkers from Nonpreeclampsia vs Preeclampsia ResponseScreeningMarkerPValueFDR PValueFDR LogWorthCLEC4A0.00402.40SYND10.00402.40PlGF0.00402.40PGF0.0123...
PUM
| Property | Measurement | Unit |
|---|---|---|
| blood pressure | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com