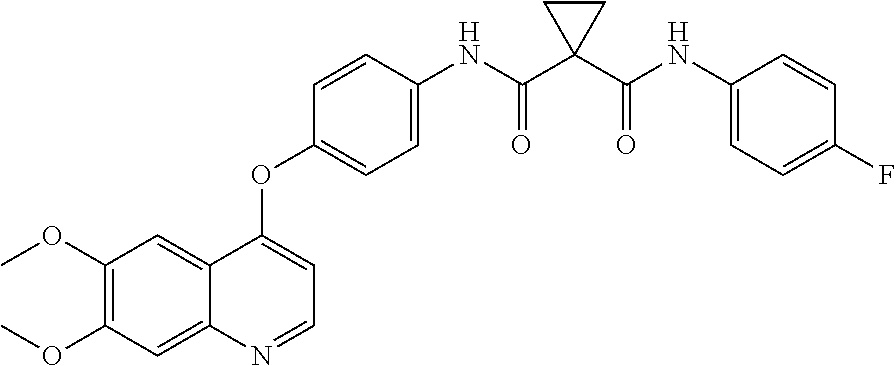
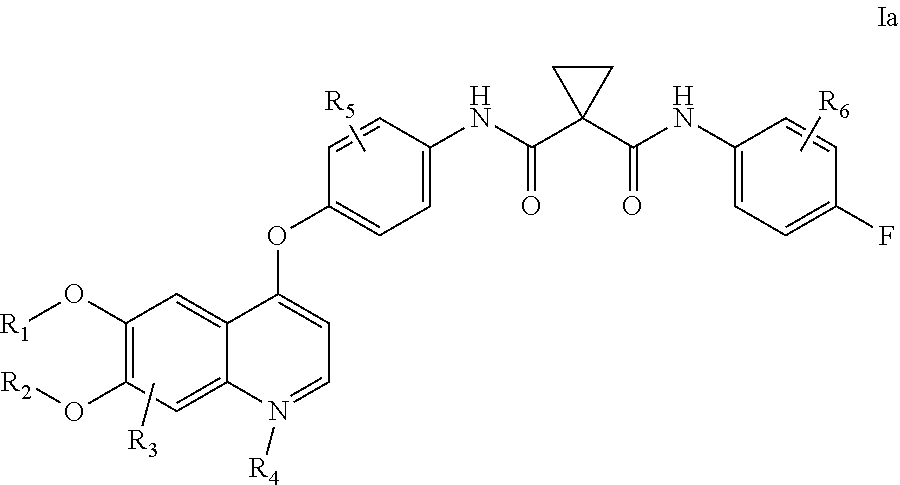
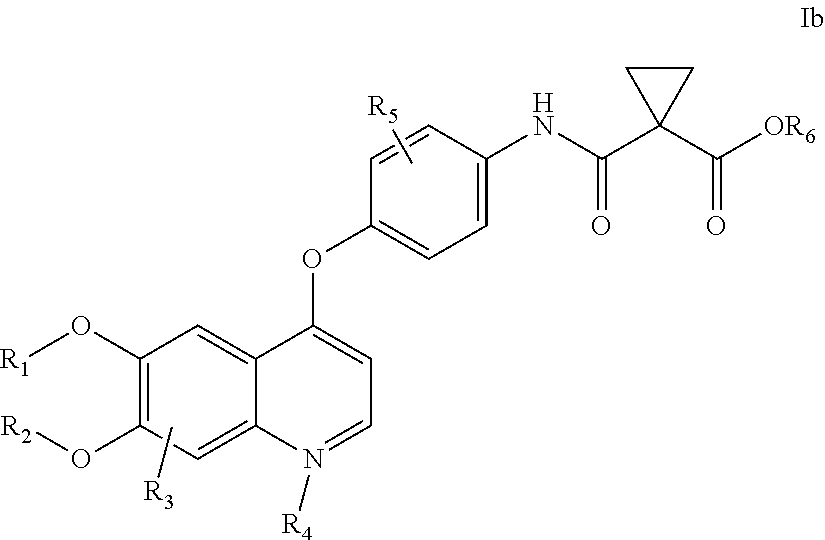
Dosing of Cabozantinib Formulations
a technology of cabozantinib and formulation, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, sexual disorders, etc., can solve problems such as cellular properties being altered, and achieve the effect of pharmacokinetic and pharmacodynamic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
Embodiment 1
[0110]A pharmaceutical formulation comprising a physiologically effective amount of cabozantinib, wherein oral administration of said composition to a selected human subject group produces in said selected human subject group:
[0111]an average cabozantinib plasma area under the curve (average AUC) of at least 60,000 ng·h / mL per each 140 mg dosage of cabozantinib delivered;
[0112]an average maximum cabozantinib blood plasma concentration (average Cmax) of at least 1000 ng / mL per each 140 mg dosage of cabozantinib delivered; and
[0113]a ratio of AUC(cabozantinib) to the AUC of the sum of cabozantinib plus measured cabozantinib metabolites:
AUC(Cabozantinib) / AUC(Cabozantinib+Measured Metabolites)
of at least 0.25;
[0114]wherein the AUC is measured from time zero to the time of last measurable concentration.
embodiment 2
[0115]A pharmaceutical formulation comprising a physiologically effective amount of cabozantinib, wherein oral administration of said pharmaceutical formulation to a selected human subject group produces in said selected human subject group:
[0116]a steady state area under the curve for cabozantinib (average AUC) of at least 35,000 ng·h / mL for a formulation comprising 100 mg of cabozantinib delivered once daily;
[0117]an average maximum cabozantinib blood plasma concentration (average Cmax) of at least 2400 ng / mL per each 100 mg dosage of cabozantinib delivered;
[0118]an average minimum cabozantinib blood plasma concentration (average Cmax) of at least 1100 ng / mL per each 100 mg dosage of cabozantinib delivered; and
[0119]a ratio of AUC(cabozantinib) to the AUC of the sum of cabozantinib plus measured cabozantinib metabolites:
AUC(Cabozantinib) / AUC(Cabozantinib+Measured Metabolites)
of at least 0.35;
[0120]wherein the AUC is measured on day 22.
embodiment 3
[0121]The pharmaceutical formulation of embodiments 1-2, wherein the measured metabolites are selected from the group consisting of:
wherein GA is a glucuronic acid moiety,
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| chemical structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com