Stabilization and isolation of extracellular nucleic acids
a nucleic acid and stabilization technology, applied in the field of stabilization and isolation of extracellular nucleic acids, can solve the problems of difficult to remove all cells, difficult to obtain essentially cell-free fractions of samples, and difficult to obtain essentially cell-free fractions, etc., to achieve stable extracellular nucleic acid population, reduce risk, and efficiently isolated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
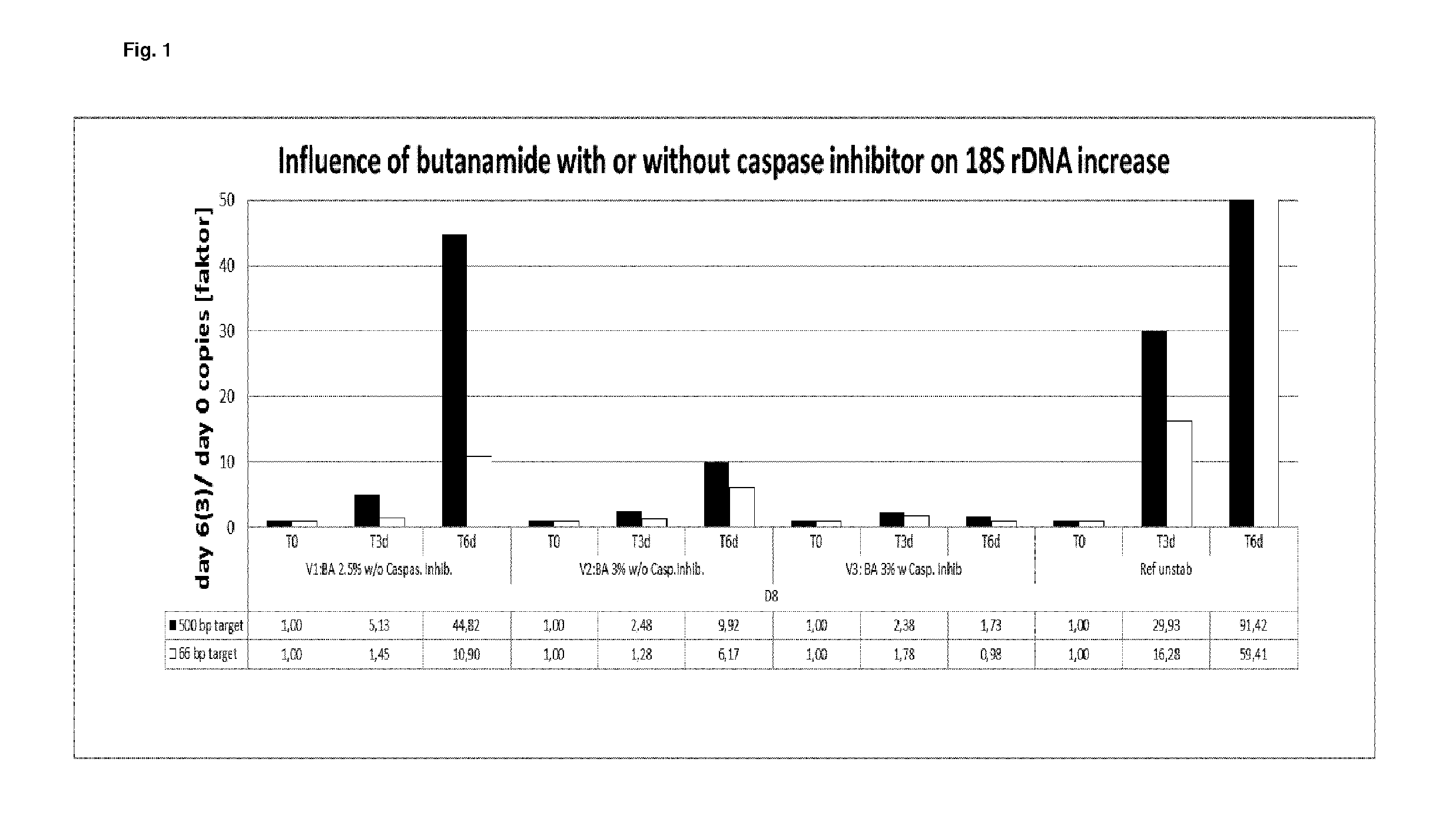
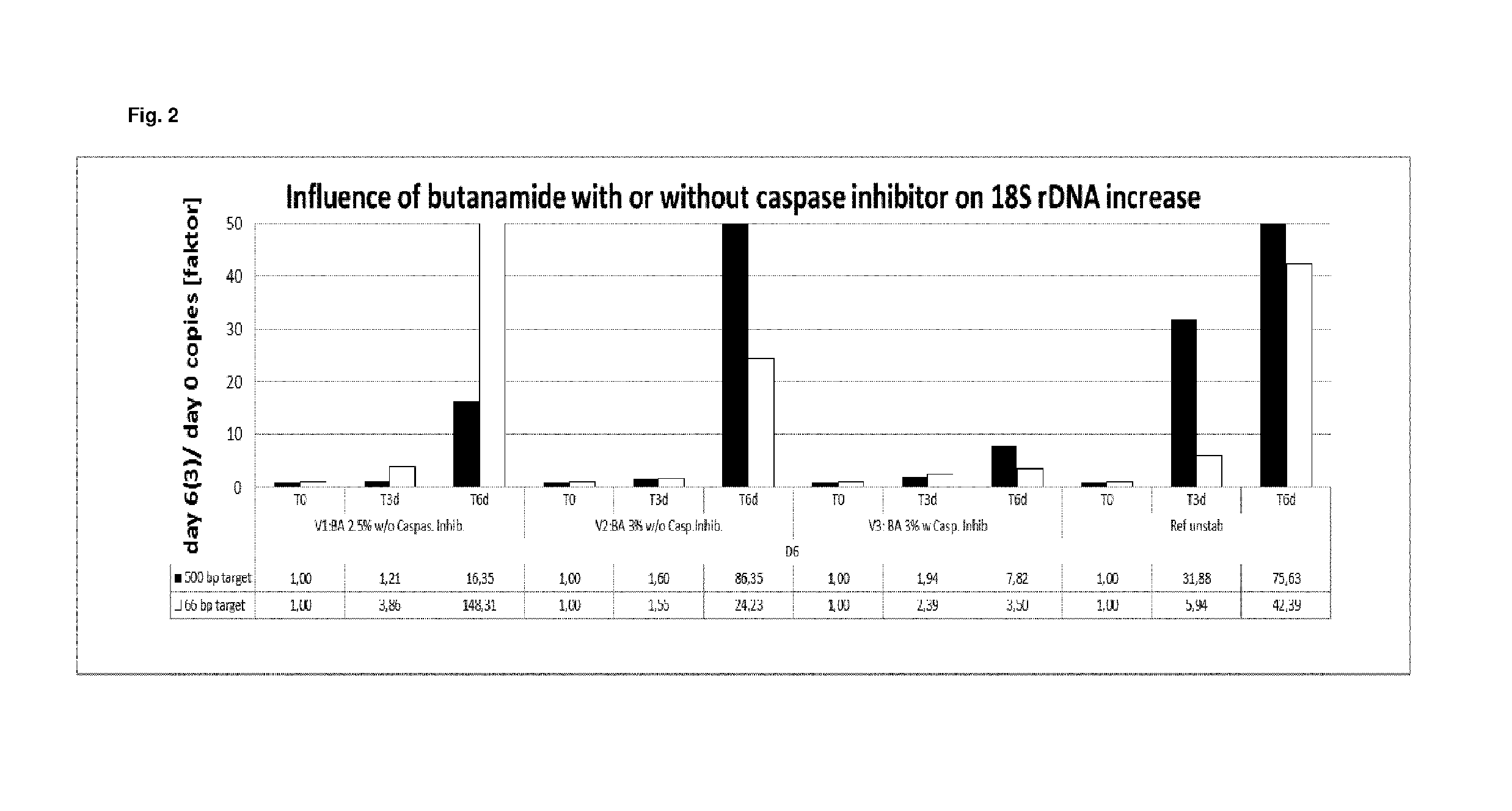
[0346]In example 1, the stabilization effect of butanamide either alone or in combination with a caspase inhibitor on EDTA stabilized blood samples was tested and compared to EDTA stabilized blood as reference.
Blood Collection and Stabilization
[0347]Blood from two different donors was collected into 10 ml K2 EDTA tubes (BD). 4.5 ml of the respectively collected blood was mixed with 0.9 ml of two different stabilization solutions A and B. Said stabilization solutions contained (per ml of stabilization solution):
A: 34.2 mg K2 EDTA and 0.15 g or 0.18 g butanamide in water When using stabilization solution A, the following final concentration in the blood / stabilization mixture was obtained: 7.2 mg K2 EDTA / ml and 2.5% (w / v) or 3% (w / v) butanamide.
B: 34.2 mg K2 EDTA, 0.18 g butanamide and 1.2 μl Quinoline-Val-Asp-CH2-OPH (caspase inhibitor) solution (1 mg dissolved in 388 μl DMSO). When using stabilization solution B, the following final concentration in the blood / stabilization mixture wa...
example 2
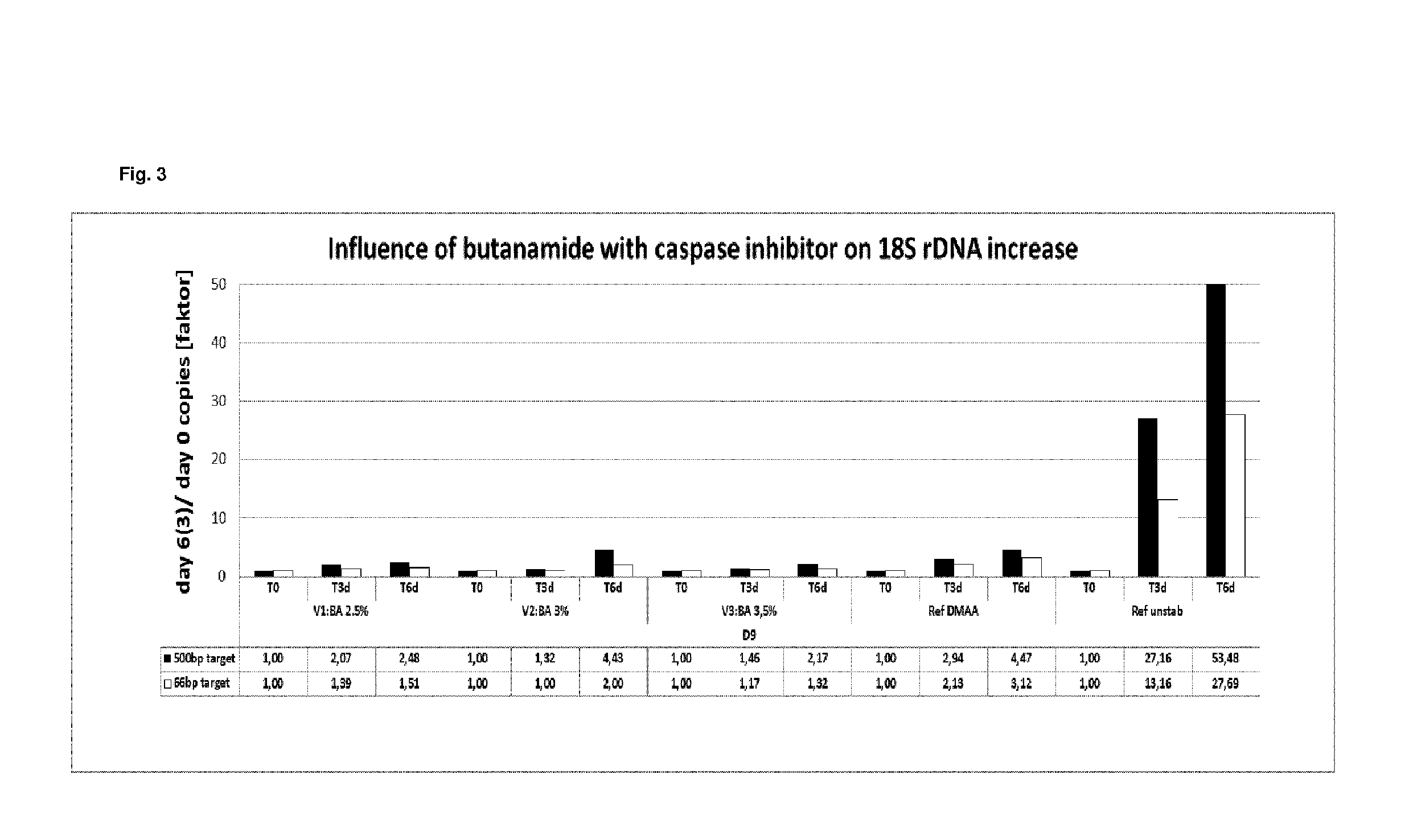
[0354]In example 2, the stabilization effect of butanamide in combination with a caspase inhibitor on EDTA stabilized blood samples was tested and compared to the stabilization effect of a caspase inhibitor in combination with N,N-dimethylacetamide (DMAA) and EDTA stabilized blood as reference.
Blood collection and stabilization Blood from four different donors was collected into 10 ml K2EDTA tubes (BD). 4.5 ml blood was mixed with 0.9 ml of different stabilization solutions containing (per ml of stabilization solution):[0355]34.2 mg K2EDTA[0356]1.2 μl Quinoline-Val-Asp-CH2-OPH (caspase inhibitor) solution (1 mg dissolved in 388 μl DMSO)[0357]0.15 g, 0.18 g or 0.21 g butanamide or 0.3 ml DMAA, respectively.
[0358]Thereby, the following final concentrations in the blood / stabilization mixtures were obtained:[0359]7.2 K2EDTA / ml[0360]1 μM Quinoline-Val-Asp-CH2-OPH (caspase inhibitor)[0361]2.5, 3 or 3.5% (w / v) butanamide or 5% (v / v) DMAA, respectively.
[0362]All stabilized blood samples wer...
example 3
[0368]In example 3, the stabilization effect of butanamide, a caspase inhibitor and N,N-dimethylpropanamide (DMPA) on EDTA stabilized blood samples was tested and compared to the stabilization effect of a caspase inhibitor in combination with N,N-dimethylacetamide (DMAA) and EDTA blood as reference.
Blood Collection and Stabilization
[0369]Blood collection and stabilization was generally performed as described in example 2, however, using different stabilization solutions. The following final concentrations in the blood / stabilization mixtures were set up[0370]7.2 mg K2EDTA / ml (all samples)[0371]1 μM Quinoline-Val-Asp-CH2-OPH (caspase inhibitor) (all samples) and[0372]0.5% (w / v) butanamide and 2.5% (v / v) DMPA; or[0373]1.5% (w / v) butanamide and 1.5% (v / v) DMPA; or[0374]2% (w / v) butanamide and 1% (v / v) DMPA; or[0375]5% (v / v) DMAA.
Extracellular Nucleic Acid Isolation and Analysis
[0376]Plasma was prepared and extracellular nucleic acids were isolated and analysed as described in example 1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com