Treating type i and type ii diabetes
a type i and diabetes technology, applied in the field of diabetes treatment methods and materials, can solve the problems of life-threatening hypoglycemia, fatal hypoglycemia, and rare achievement, and achieve the effects of reducing or avoiding fatal hypoglycemia, reducing or eliminating fatal hypoglycemia, and improving quality of li
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Insulin Gene Therapy for Diabetes
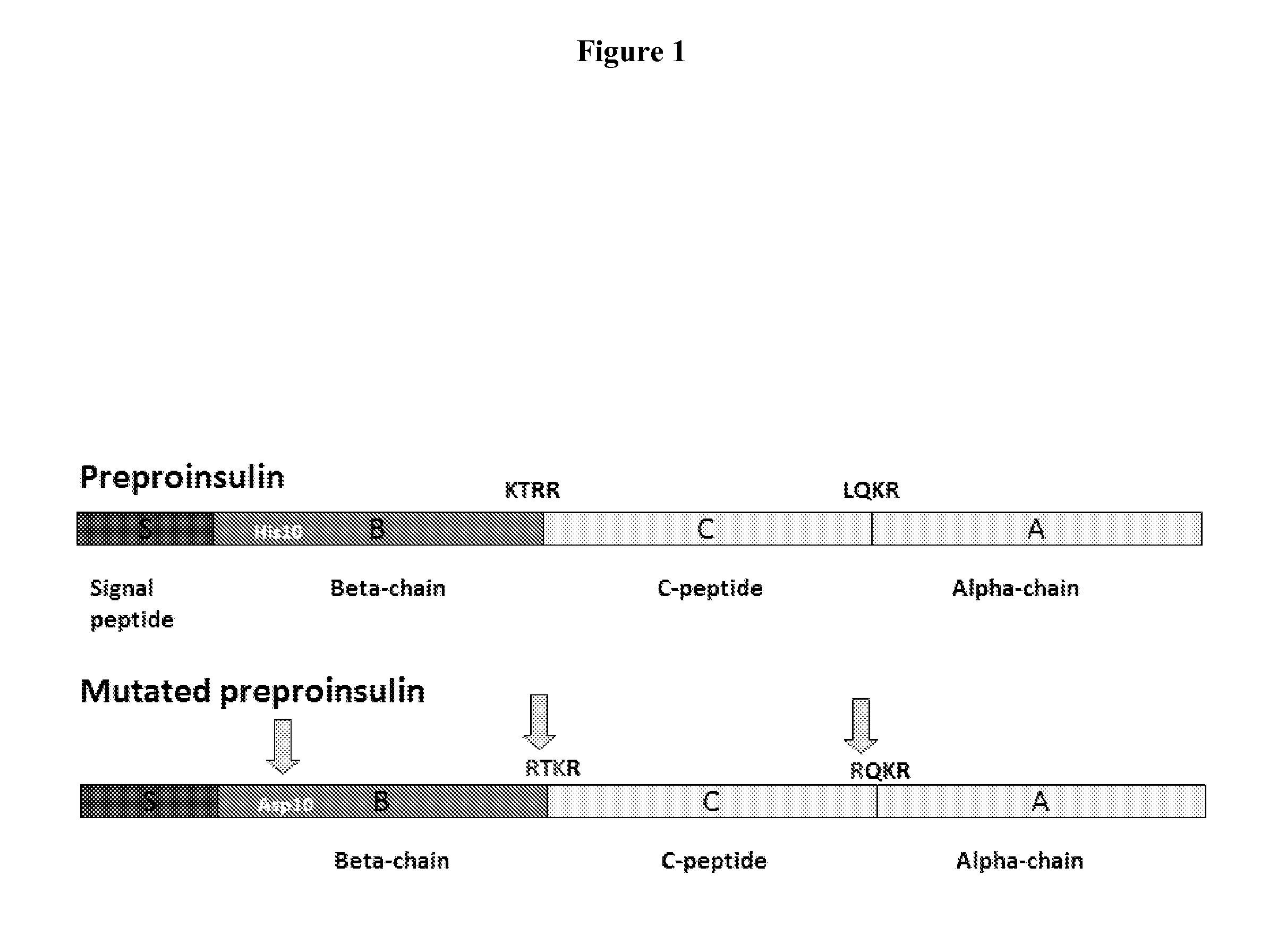
[0034]Nucleic acid encoding human preproinsulin was designed such that the proprotein convertase cleavage signals at the B / C and C / A peptide junctions were modified to allow recognition and cleavage by furin, which is present in the Golgi compartment of most mammalian cells. Briefly, the B / C and C / A cleavage signals were mutated, respectively, from KTTR to RQKR and from LQKR to RQKR (FIG. 1). As a result of these modifications, the expressed preproinsulin polypeptide is processed to functional insulin in the Golgi apparatus of most mammalian cell types and then released by the cell into the extracellular medium.
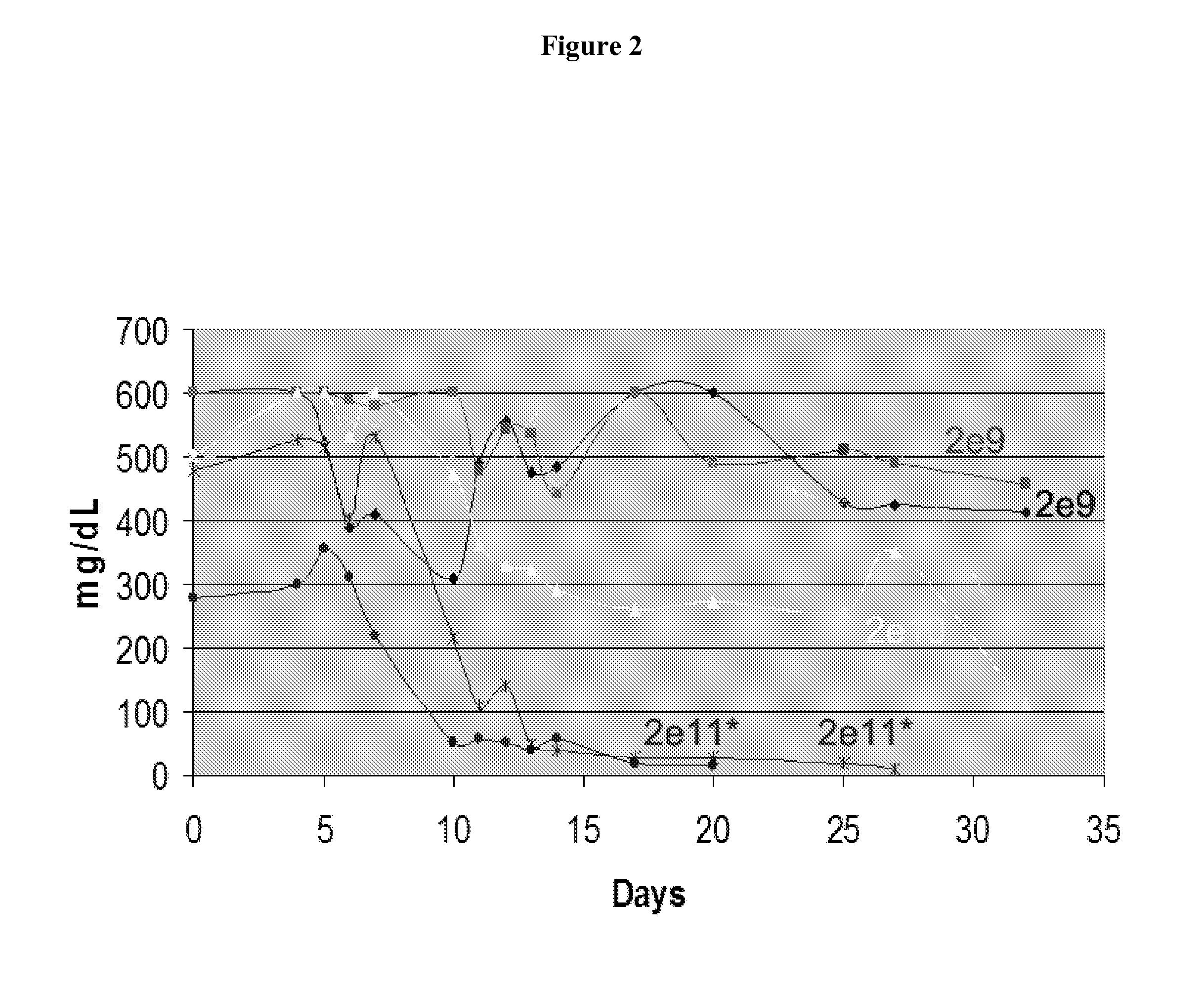
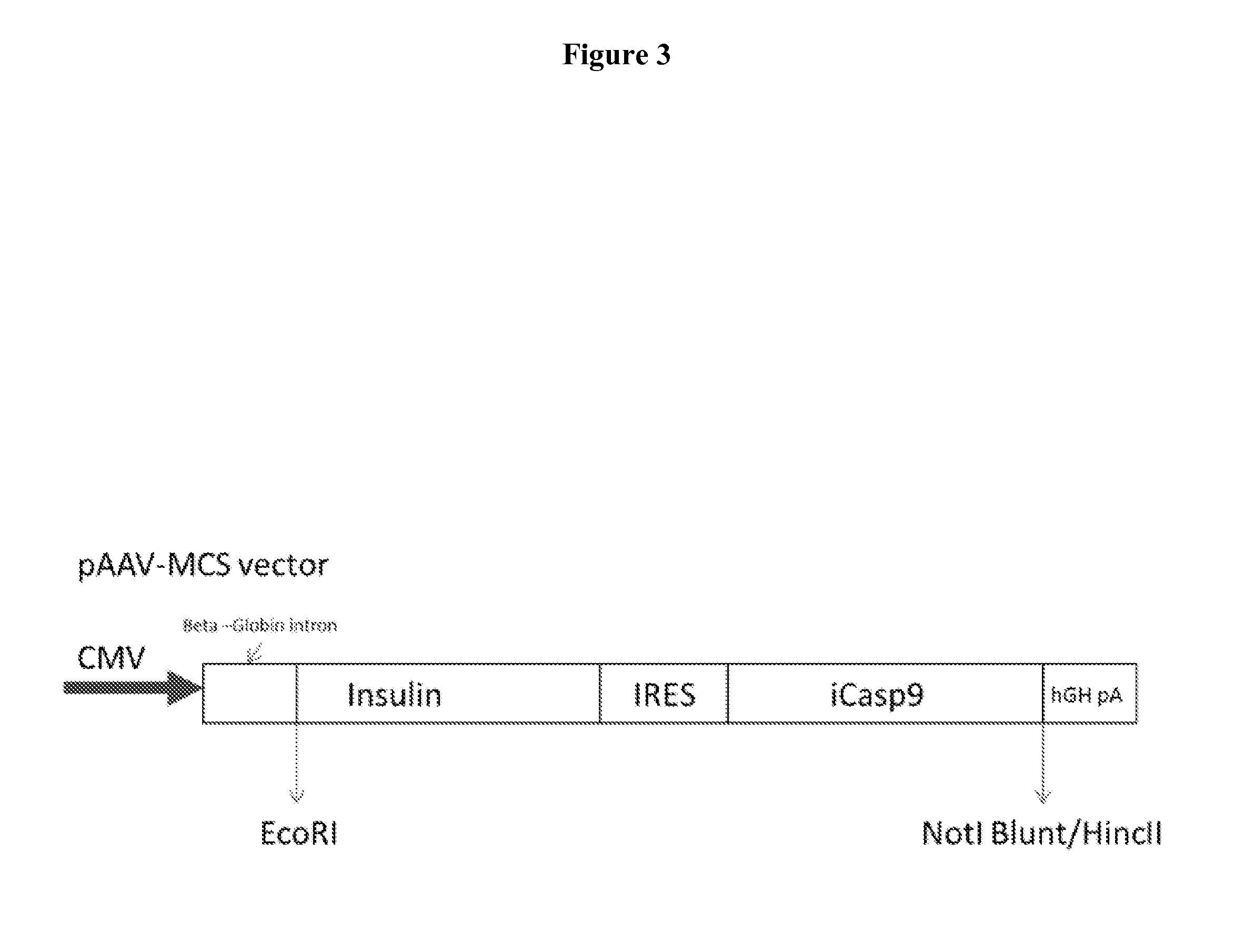
[0035]Initially, AAV1 or sleeping beauty (SB) vectors encoding a furin-activatable proinsulin without an iC9 death switch were used (FIG. 1). Intramuscular administration of the AAV1-insulin vector to mice with streptozotocin-induced diabetes led to a dose-dependent reduction in the blood glucose level, but caused fatal hypoglycemia at higher ...
example 2
Safety Studies of AAV-insulin-iC9 in Mice
[0039]The optimal vector dose is determined in mice. Experimental diabetes is induced in 8-week-old C57B1 / 6 mice by multiple intraperitoneal injections of streptozotocin (50 mg / kg) resuspended in 0.1 M citrate buffer (pH 4.5) over the course of five days. Fasting blood glucose levels are monitored bi-weekly by FreeStyle Lite Blood Glucose Monitor (Abbott, Ill., USA). Two weeks after diabetes induction, groups of diabetic mice (n=6) receive increasing doses of AAV1-INS-iCasp9 (2.5×1011, 5×1011, 1×1012, 2×1012, 4×1012, 8×1012, 1.6×1013, and 3.2×1013 vg / kg in 200 μL of saline) distributed into tibialis cranealis, gastrocnemius and quadriceps muscles of both hindlimbs. Control groups are non-STZ-treated and STZ-treated mice treated with saline. The mice are monitored for body weight, plasma insulin, c-peptide, and blood glucose levels in the morning and evening and glucose responsiveness through glucose challenge for four weeks after AAV1 adminis...
example 3
Safety Studies of AAV-insulin-iC9 in Non-Human Primates
[0043]Cynomolgus macaques are exposed to streptozotocin (STZ) for diabetes induction followed by disease characterization and then application of AAV1-insulin-iC9 gene therapy for safety and efficacy evaluations. All animals are euthanized at the completion of study and undergo full necropsy. In both the pre and post-STZ phase, animals are screened for baseline characteristics including CBC, chemistry (CPK inclusion required), and HA1c. Blood glucose is measured at least twice daily in the morning (pre prandial) and evening (post prandial). A metabolic panel including c-peptide, IVGTT, AST, MMTT, and 8 hour standard glucose curve is performed. AAV1-ins-IC9 is administered by multiple intramuscular injections unilaterally in the leg muscle under general anesthesia. Dosing is as follows (in genome copies per kilogram): 10e12 (NHP#1), 2×10e12 (NHP#2), or 4×10e12 (NHP#3) using a Quadra-Fuse needle (or equivalent) injection device at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com