Co-agonists of the glucagon and glp-1 receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
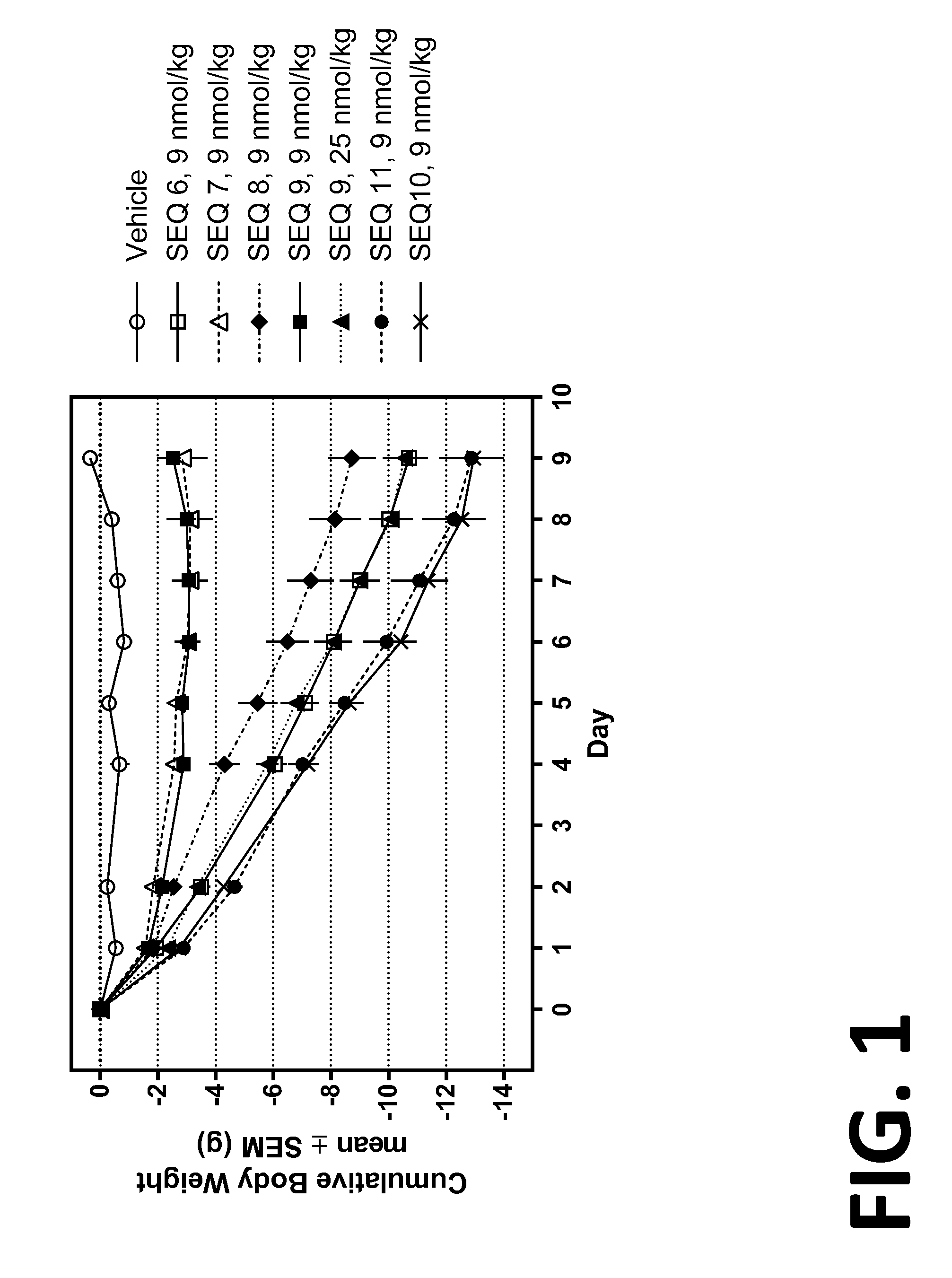
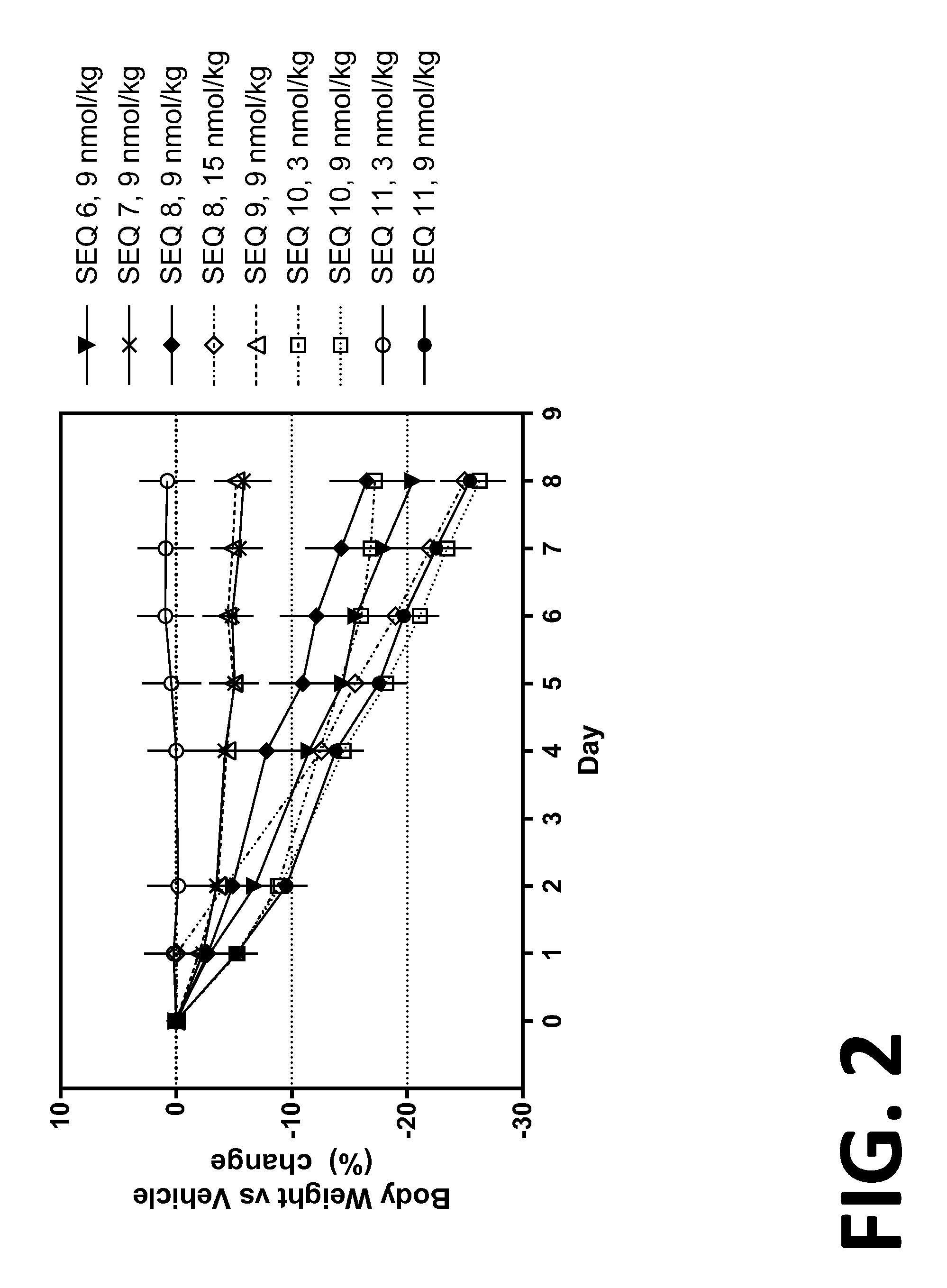
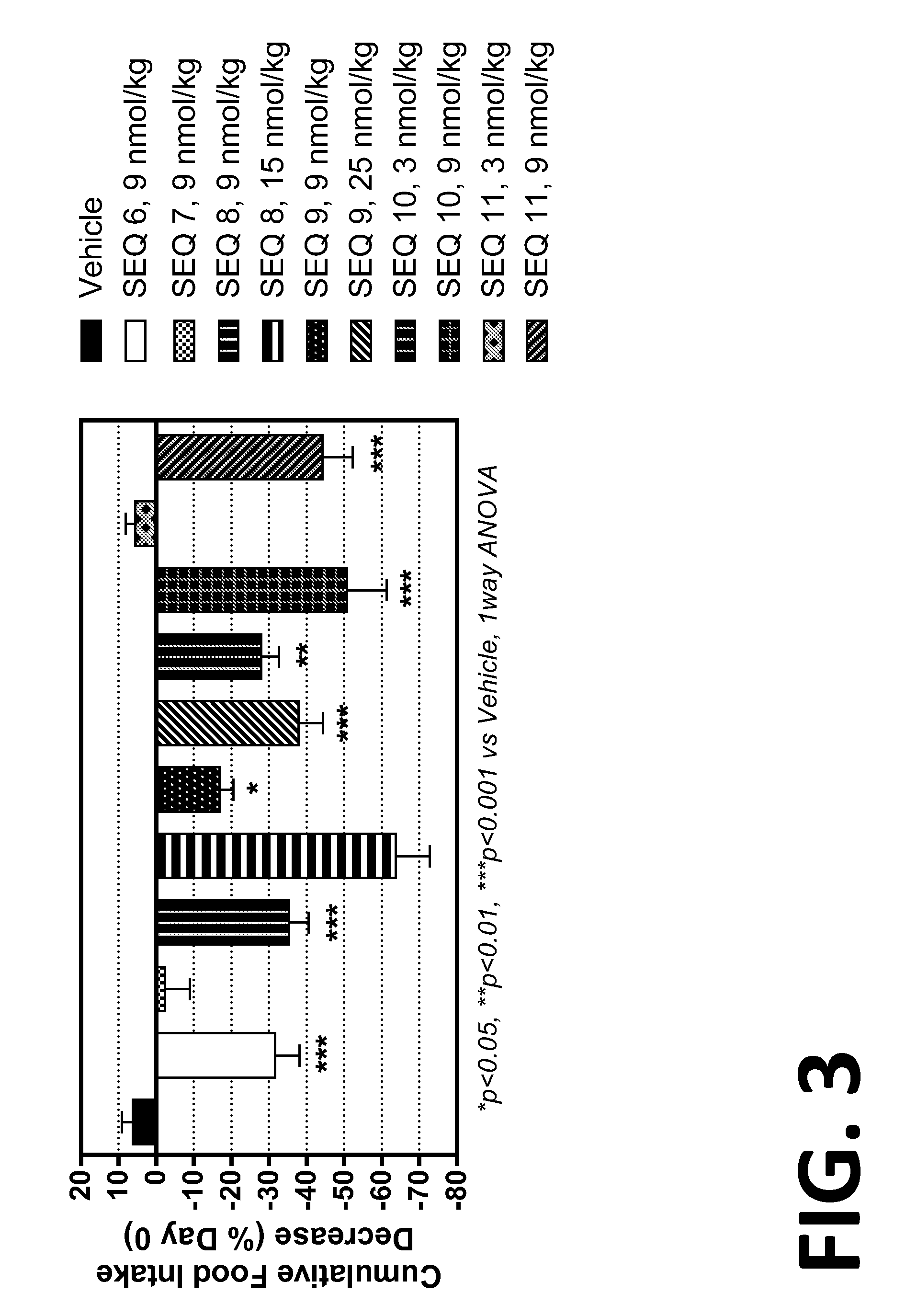
[0261]Procedure for the synthesis of Co-agonist Peptide 1: His1-D-Ser2-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Lys10(γGlu-C16)-Ser-Lys-Tyr-Leu-Asp-Ala-Arg-Ala-Ala-Gln-Asp-Phe22-Val23-Gln-Trp-Leu-Leu-Asp-Thr-CONH2 (SEQ ID NO:6) was as follows.
[0262]The peptide was synthesized on a Rink-amide PEG-PS resin, Champion (Biosearch Technologies (150 μmol, loading 0.28 mmol / g) on a Symphony Protein Technologies Inc apparatus.
[0263]All the amino acids were dissolved at a 0.3 M concentration in a solution of 0.3M HOBt (Hydroxybenzotriazole) in DMF. The acylation reactions were performed for 1 hour with 5-fold excess of activated amino acid over the resin free amino groups. The amino acids were activated with equimolar amounts of HATU (O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate) and a 2-fold molar excess of DIEA (diisopropylethylammine) solution 2M in NMP.
[0264]Double acylation reactions of 45 minutes were performed from His1 to Trp25. The crude peptide (130 mg in 3 ml of D...
example 2
[0266]Procedure for the synthesis of Co-agonist Peptide 2: His1-D-Ser2-Gln-Gly-Thr-Phe-Thr-S er-Asp-Lys10(γGlu-C16)-Ser-Lys-Tyr-Leu-Asp-Val-Arg-Ala-Ala-Gln-Asp-Phe22-Val23-Gln-Trp-Leu-Leu-Asp-Thr-CONH2 (SEQ ID NO:7) was as follows.
[0267]The peptide was synthesized as previously reported for SEQ ID NO: 6 / Example 1
The crude peptide (130 mg in 3 ml of DMSO) was purified by reverse-phase HPLC using preparative Waters Delta-Pak™ C-4 cartridges (40×200 mm, 15 μm, 300 Å) and using as eluents (A) 0.1% TFA in water and (B) 0.1% TFA in acetonitrile. The following gradient of eluent B was used: 30% B to 30% B over 5 min, 50% B to 50% B over 20 min-80% B, flow rate 80 mL / min, wavelength 214 nm. Yield 16%, 95% pure.
[0268]The final peptide was characterized on an Acquity UPLC Waters Chromatograph, with BEH300 C4 Acquity Waters 2.1×100 mm, 1.7 μm, at 45° C., using H2O, 0.1% TFA (A) and CH3CN, 0.1% TFA (B) as solvents and also characterized by electrospray mass spectrometry on a Acquity SQ Detector...
example 3
[0269]Procedure for the synthesis of Co-agonist Peptide 3: His1-D-Ser2-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Lys10(γGlu-γGlu-C16)-Ser-Lys-Tyr-Leu-Asp-Val-Arg-Ala-Ala-Gln-Asp-Phe22-Val23-Gln-Trp-Leu-Leu-Asp-Thr-CONH2 (SEQ ID NO:8) was as follows.
[0270]The peptide was synthesized as previously reported for Co-agonist Peptide 1. The crude peptide (130 mg in 3 ml of DMSO) was purified by reverse-phase HPLC using preparative Waters Delta-Pak™ C-4 cartridges (40×200 mm, 15 μm, 300 Å) and using as eluents (A) 0.1% TFA in water and (B) 0.1% TFA in acetonitrile. The following gradient of eluent B was used: 30% B to 30% B over 5 min, 30% B to 60% B over 20 min-80% B, flow rate 80 mL / min, wavelength 214 nm. Yield 20%, >90% pure.
[0271]The final peptide was characterized on an Acquity UPLC Waters Chromatograph, with BEH300 C4 Acquity Waters 2.1×100 mm, 1.7 μm, at 45° C., using H2O, 0.1% TFA (A) and CH3CN, 0.1% TFA (B) as solvents and the following gradient: 35% to 65% B in 4 min, flow 0.4 mL / min. The pept...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com