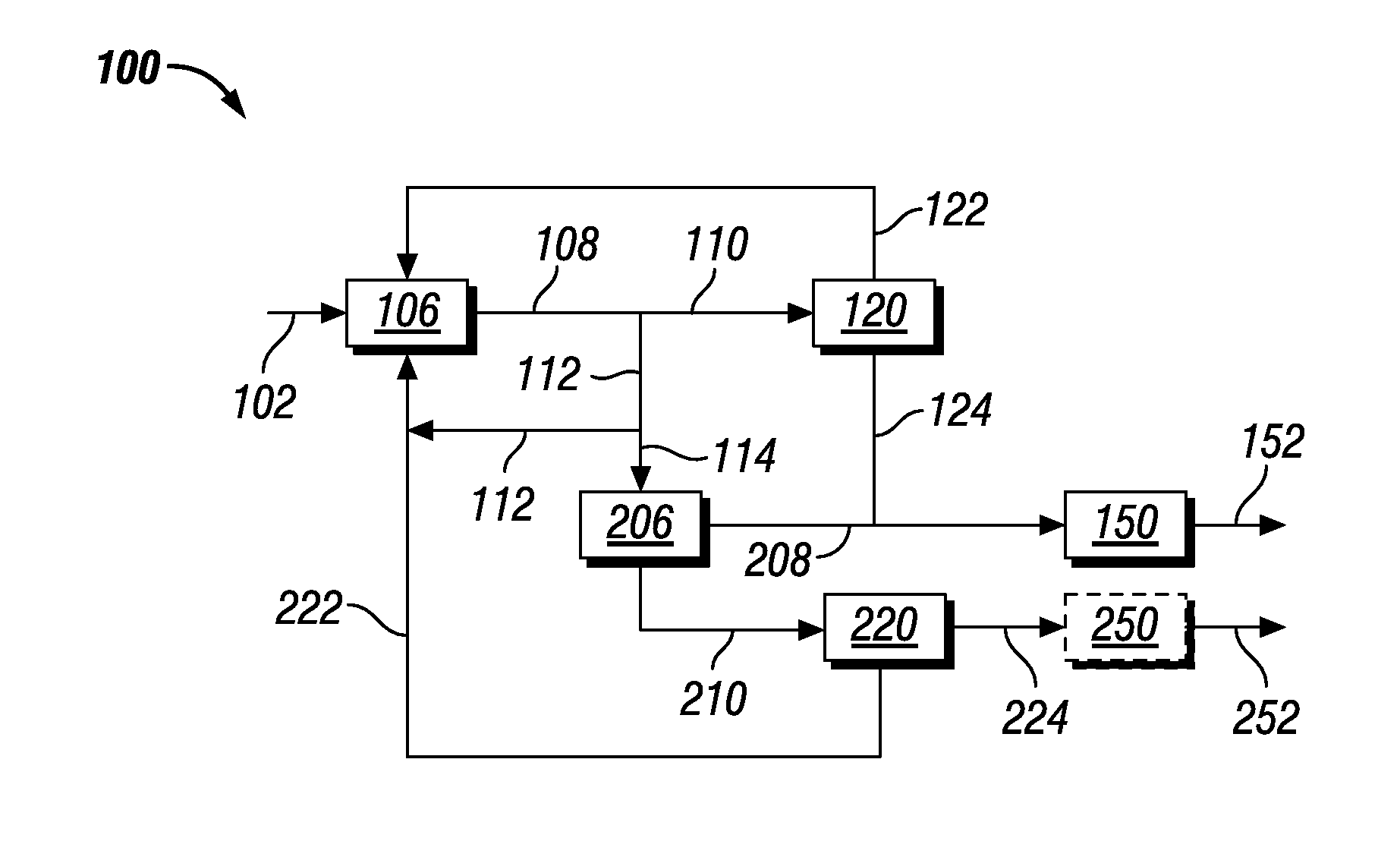
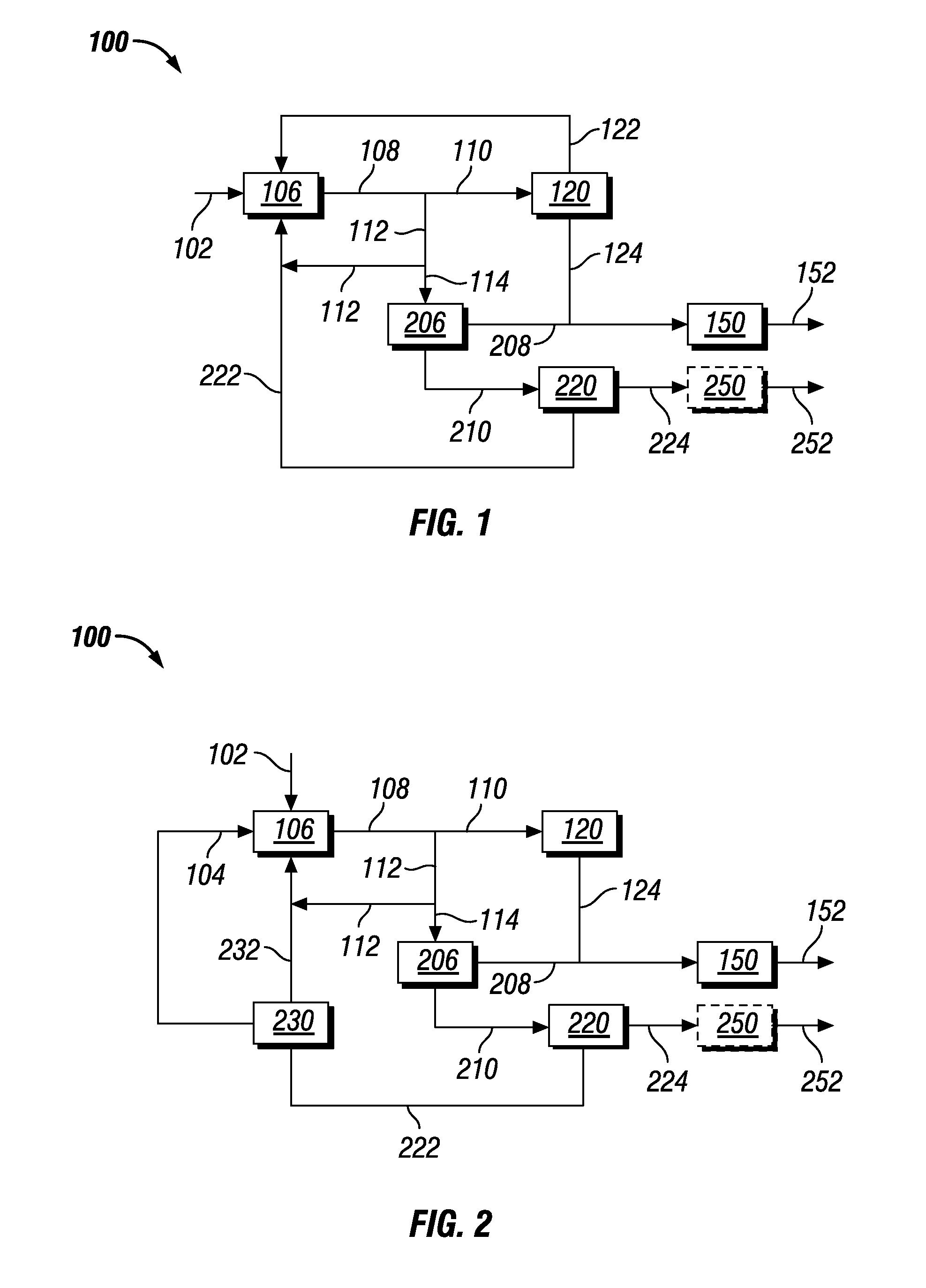
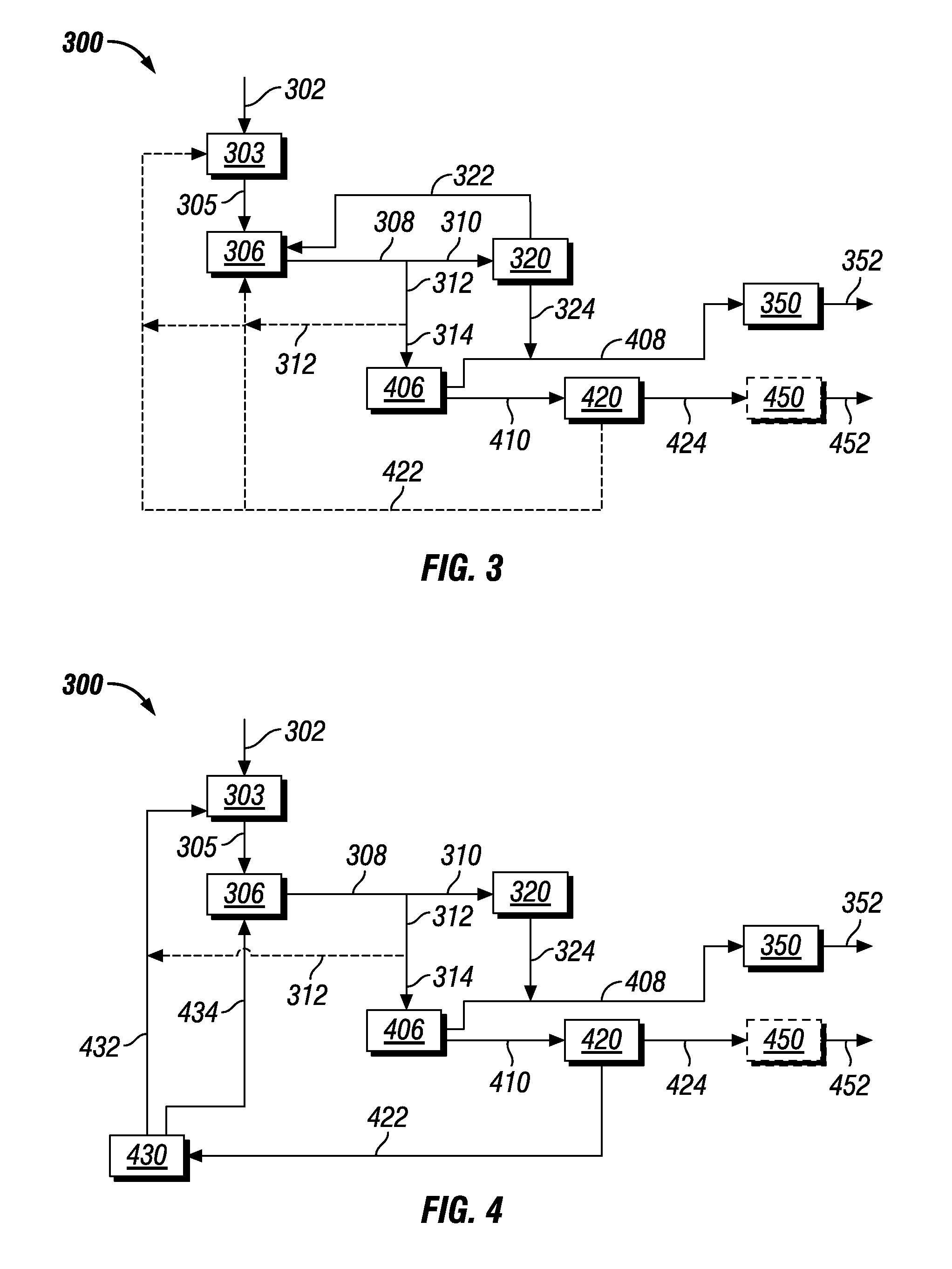
Co-production of biofuels and glycols
a biofuel and glycol technology, applied in the preparation of oxygen-containing compounds, fuels, oh group elimination, etc., can solve the problems of limited supply, high yield of glycols, and inability to convert carbohydrates to glycols at high conversion rates of feed carbohydrates, etc., and achieve high yields of glycols.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Flow Reaction with Sorbitol Feed
[0140]A ¼-inch diameter flow reactor was packed with 1.26 grams of 1.9% Pt / zirconia modified with rhenium at Re:Pt rato of 3.75:1 prepared according to the method in Example 7 of US2008 / 0215391. After reduction under H2 flow at 400° C., the reactor was purged with N2 to establish a backpressure of 59.7 bar of nitrogen. Reactor temperature was reduced to 250° C., and flow of 50 wt % sorbitol feed in deionized water was established at a weight hourly space velocity of 0.98 grams of feed per gram of catalyst per hour. HPLC analysis of reactor effluent indicated formation of ethylene glycol and propylene glycol at a yield of 7.1 wt %, relative to sorbitol converted. Increase in space velocity to 1.91 / h led to a decrease in yield of EG and PG to 5.6 wt %, relative to the mass of sorbitol converted. The remainder of conversion corresponded primarily to formation of monooxygenates, with retention time less than that of sorbitol, when analyzed via the DB5-ox ...
example 2
Selectivity with Cysteine
[0141]Example 1 was repeated with 1% cysteine present in the 50 wt % sorbitol feed. Yield of ethyene glycol and propylene glycol was increased to 10.9 wt % relative to the mass of sorbitol converted. This example demonstrates enhanced selectivity to glycols at lower conversions, in the presense of a reaction inhibitor such as cysteine.
example 3
Flow Reaction
[0142]Example 1 was repeated at 230° C. Sorbitol conversion of 76% was observed, with a yield of ethylene glycol and propylene glycol of 7.0%, relative to the mass of sorbitol converted.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| steady state temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com