Production of polyhydroxyalkanoates with a defined composition from an unrelated carbon source
a technology of monomeric composition and polyhydroxyalkanoate, which is applied in the direction of lyase, ligase, enzymology, etc., can solve the problems of low-cost sources of such monomers or monomer precursors, the inability to produce mcl-pha at high yields from an unrelated carbon source, and the inability to meet the requirements of specific commercial applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Summary
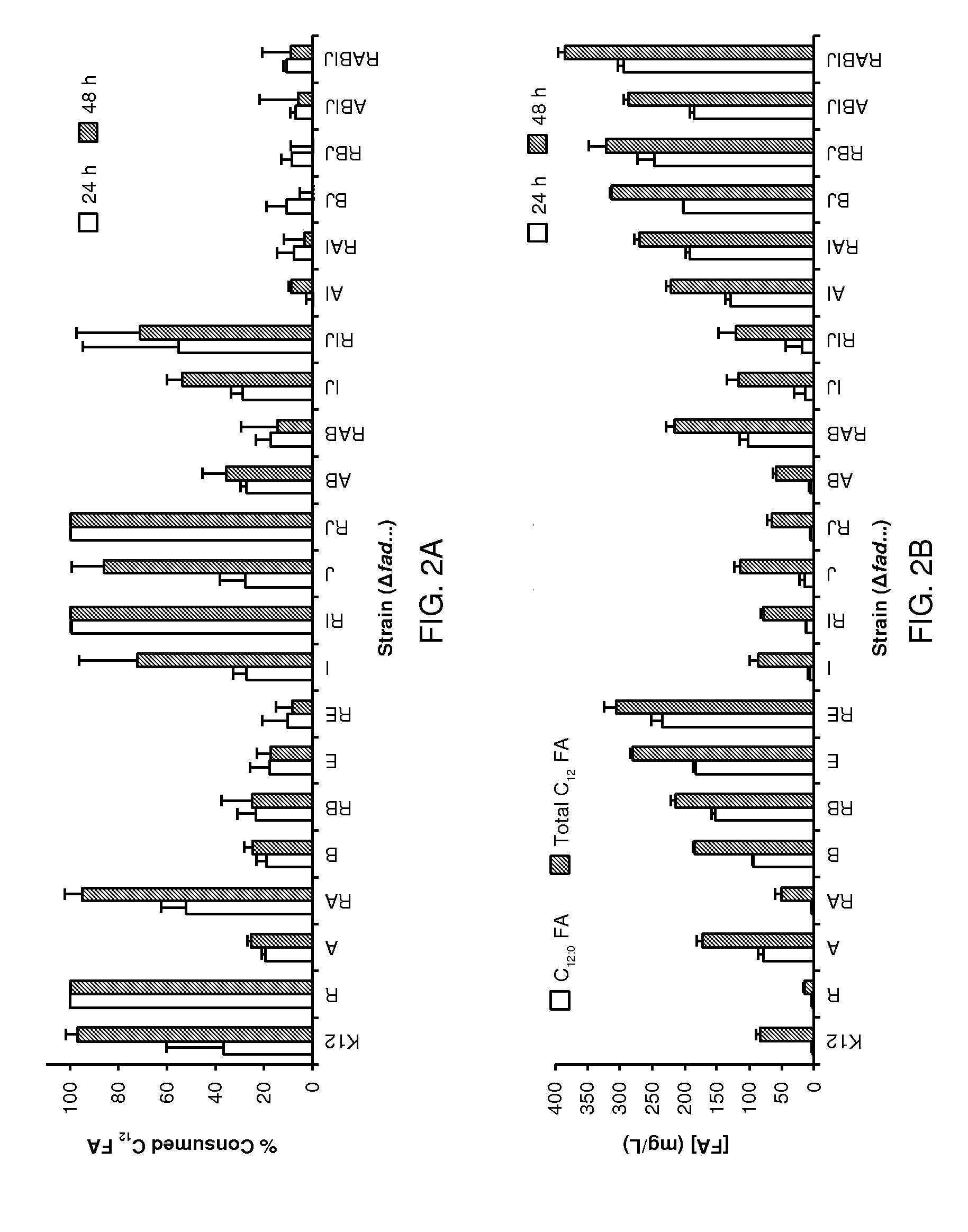
[0138]The following Examples present a rational approach for producing mcl-PHA homopolymer from an unrelated carbon source (i.e., glucose) in E. coli. A characterization of a panel of mutant E. coli strains to determine the impact of β-oxidation enzymes on fatty acid consumption and mcl-PHA synthesis is presented. A characterization of two PHA synthases (PhaC) and four enoyl-CoA hydratases (PhaJ) for producing mcl-PHA in E. coli, thereby identifying a suitable combination for making mcl-PHA, is also presented. An examination of the impact of different modes of regulating acyl-CoA synthetases on PHA titer is shown. Finally, engineering of a strain of E. coli to produce mcl-PHA with a composition matching the product profile of the expressed thioesterase is shown. The strategy involves constructing a strain of E. coli in which key genes in fatty acid β-oxidation are deleted and BTE, phaJ3 and phaC2 from Pseudomonas aeruginosa PAO1, and PP—0763 from P. putida KT2440 are overexpr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical density | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| v/v | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com