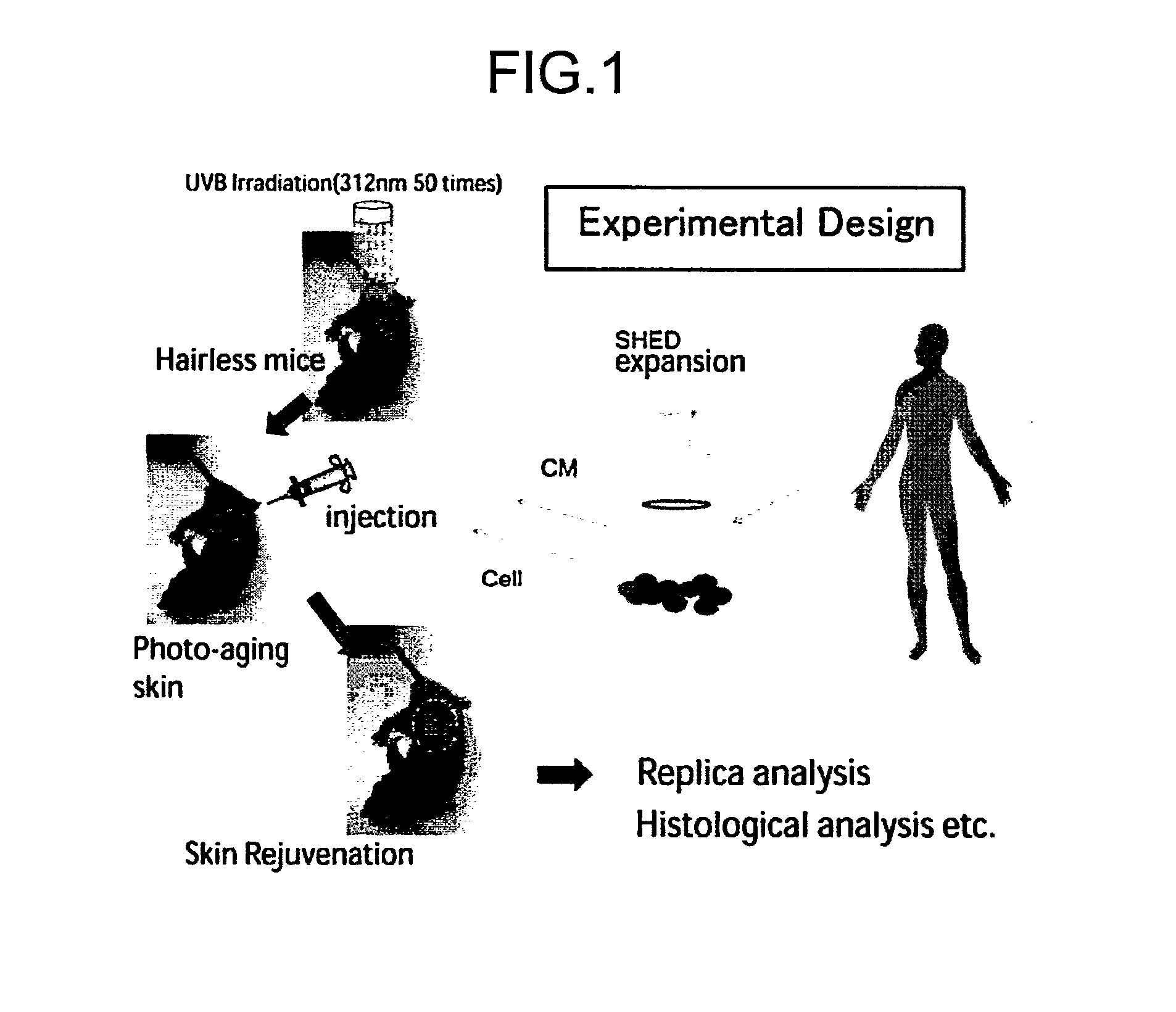
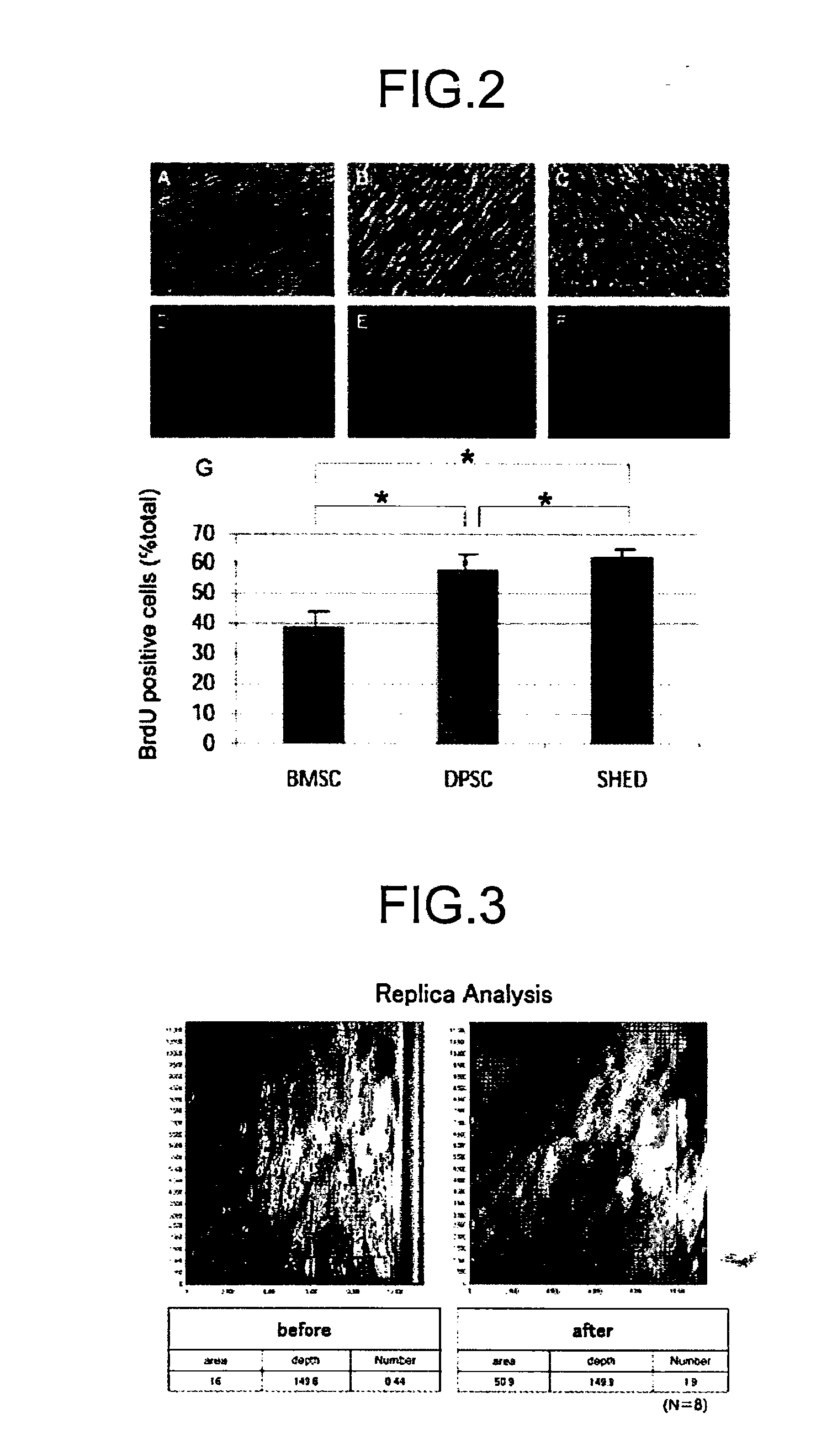
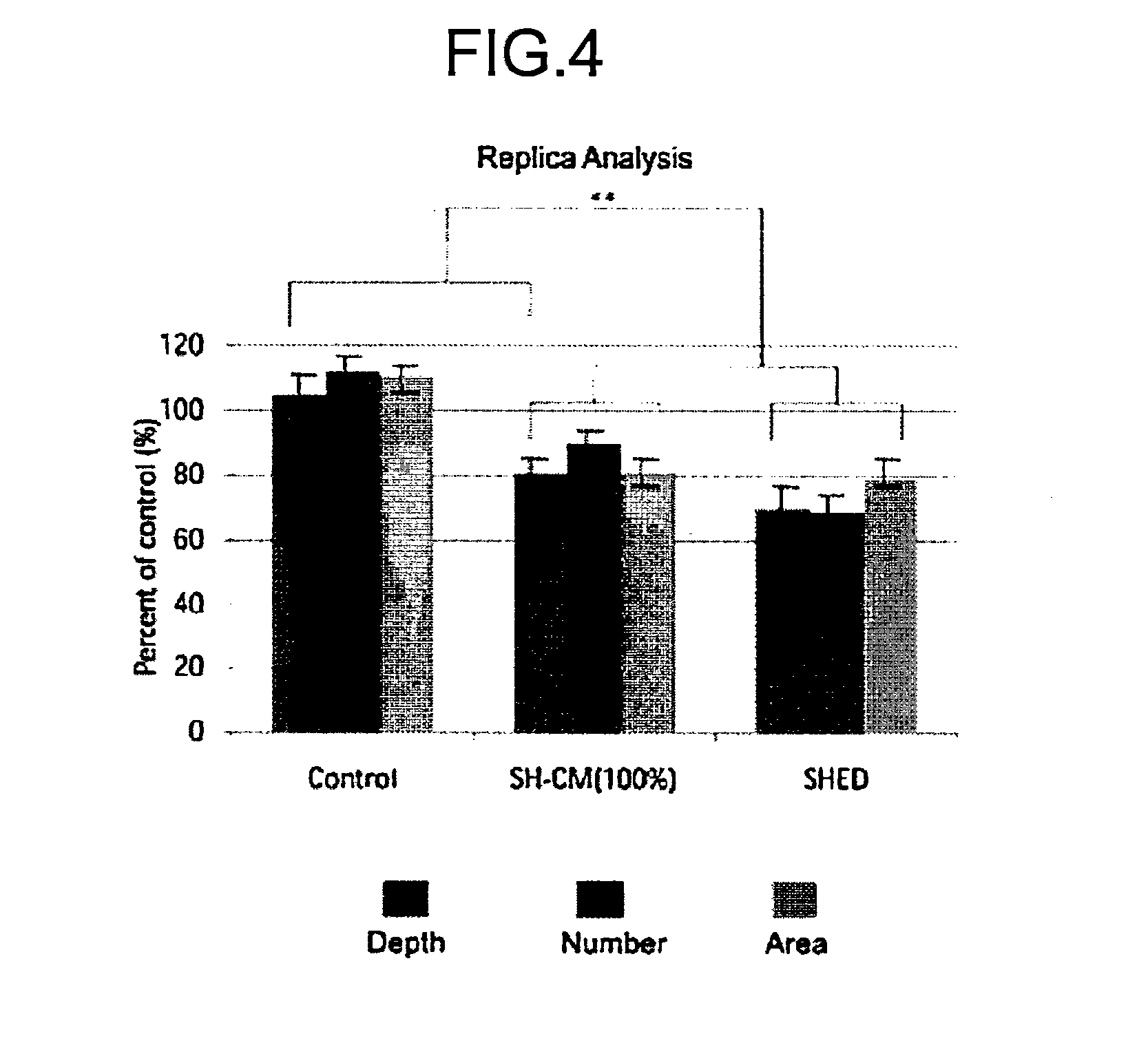
Composition for Treatment of Damaged Part
a technology for treating damaged parts and parts, applied in the direction of skeletal/connective tissue cells, peptide/protein ingredients, peptide sources, etc., can solve the problems of morality and safety serious problems, the number and proliferation and differentiation potential of bone marrow stem cells (bmscs) decline with increasing, and the inability to perform invasive and painful procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0233](1) Subjects and Cell Cultures
[0234]Human dental pulp tissues were obtained from clinically healthy extracted deciduous teeth and permanent teeth from eight patients. These experimental protocols were approved by the ethics committee of Nagoya University. SHED and DPSCs were isolated and cultured as described in Proc Natl Acad Sci USA 2000; 97: 13625-30 or Proc Natl Acad Sci USA 2003; 100: 5807-12.
[0235]Briefly, the pulp was gently removed and digested in a solution of 3 mg / mL collagenase type I and 4 mg / mL dispase at 37° C. for 1 hour. After filtration using 70-mm cell strainers (Falcon; BD Labware, Franklin Lakes, N.J.), cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; GIBCO, Rockville, Md.) containing 20% mesenchymal cell growth supplement (Lonza Inc., Walkersville, Md.) and antibiotics (100 U / mL penicillin, 100 mg / mL streptomycin and 0.25 mg / mL amphotericin B; GIBCO) at 37° C. with 5% CO2. After primary culture, the cells were subcultured...
example 2
(1) Preparation of Growth Factor Mixture (Powder)
[0270]Immortalized human mesenchymal stem cells (MSCs: Ronza Co., Ltd, USA) were used to prepare a growth factor (GF) admixture. The cells were cultured for 2 to 8 passages using 10% FSC-containing DMEM. At a stage of 80% confluence of cells in the culture dish, the supernatant (culture medium: CM) was sampled. The sampled CM was then added to ethanol (CM: Ethanol=1:9), and incubated at −20° C. for 60 min. The CM was concentrated by spinning (4° C., 1500 rpm, 15 min). The remaining CM was washed with 90% ethanol at −20° C., and then spun again.
[0271]The concentrated CM was freeze-dried, thereby obtaining a growth factor powder.
(2) Growth Factors in the Powder
[0272]Each growth factor in the powder was analyzed by the Western-blotting method. The detected growth factors are as follows: PDGF, VEGF, IGF, KGF, HGF and TGF.
example 3
Experimental Study of Bone Regeneration by Growth Factor
[0273](1) Methods
[0274]Four cylinder-shaped bone defects, each having a diameter of 10 mm and a depth of 10 mm, were formed in the dog mandible.
[0275]A titanium implant (3.75 mm in diameter) was inserted into the center of each defect.
[0276]The spaces around the implant were filled with graft materials, such as (1) PRP, (2) 100% GF, (3) MSCs (1×1,000,000) and (4) empty defect (control) (FIG. 10).
[0277](2) Results
[0278]Eight weeks later, the dog was euthanized and the mandible with the implants was dissected. A histological specimen was made and BIC (Bone-Implant Contact) was calculated using a light microscope and an image analyzing system (FIG. 11).
BIC=total length of bone contact to implant surface / total length of implant surface×100(%)
[0279]There was no significant difference in BIC between MSC (65.0) and GF (58.6), and the respective BIC values in MSC and GF were much higher than those of the control (26.4) and PRP (44.2) (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com