Method for controlling proliferation of cord blood hematopoietic stem cells and use thereof
a technology of hematopoietic stem cells and cord blood, which is applied in the field of controlling the proliferation can solve the problems of affecting the expression of other genes, cord blood may not provide the number of cells necessary for transplantation, and the collection of donors is a considerable burden, so as to inhibit the proliferation and differentiation, control the proliferation and differentiation of cord blood hematopoietic stem cells, and excellent safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
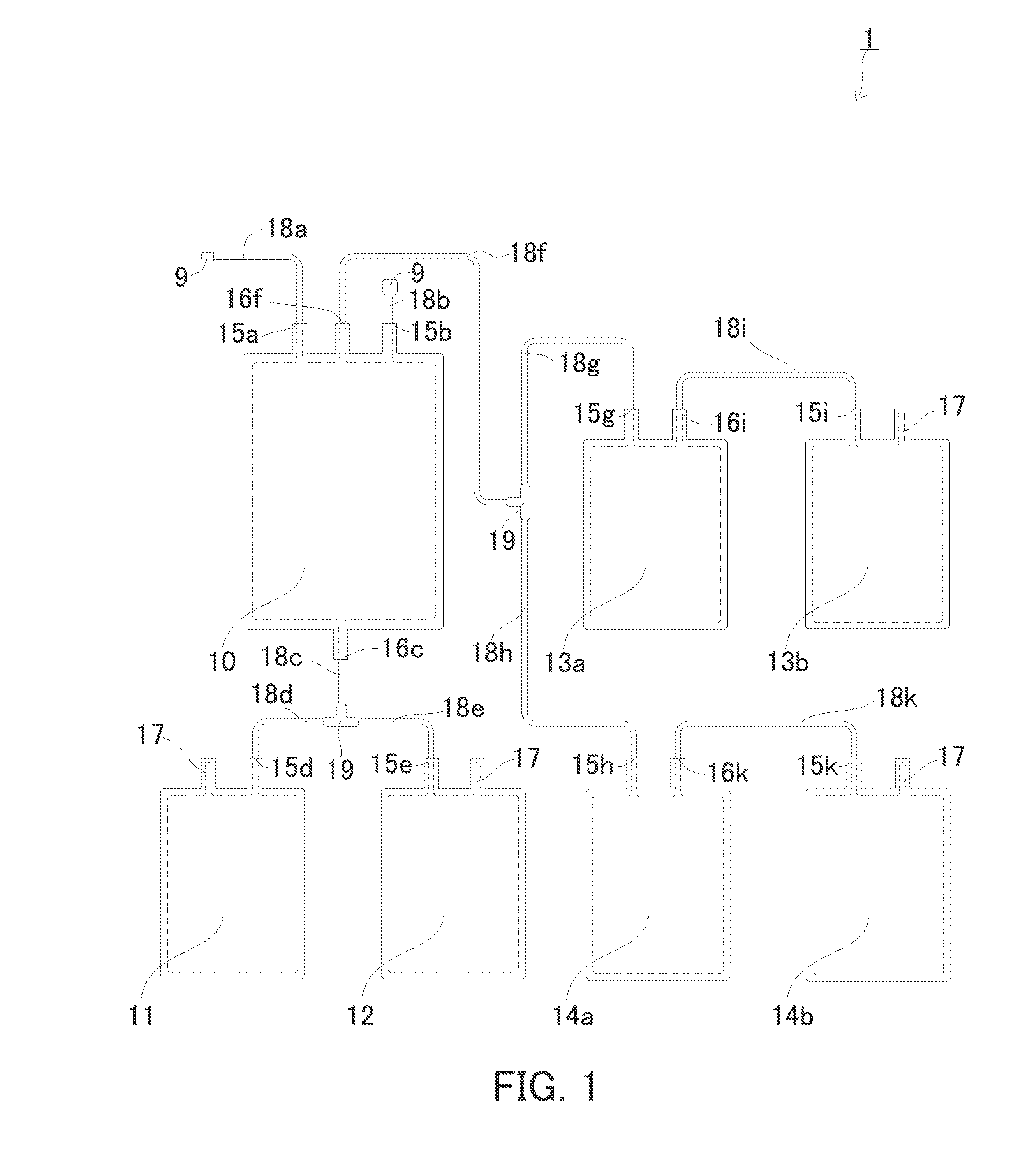
[0146]Next, an example of the first cord blood component preparation device according to the present invention will be described with reference to FIG. 1. It is to be noted, however, the present invention is not limited thereto.
[0147]FIG. 1 is a plan view schematically showing the cord blood component preparation device of the present embodiment. The cord blood component preparation device 1 includes: a storage unit 10 for storing cord blood; an erythrocyte accommodation unit 11 for accommodating erythrocytes; a hematopoietic stem cell accommodation unit 12 for accommodating hematopoietic stem cells; a first plasma accommodation unit 13a and a second plasma accommodation unit 14a for accommodating plasma; and a first serum accommodation unit 13b and a second serum accommodation unit 14b for accommodating serum. In the storage unit 10 and the respective accommodation units 11 to 14b shown in FIG. 1, the inside of the dotted line is a space capable of accommodating the respective comp...
second embodiment
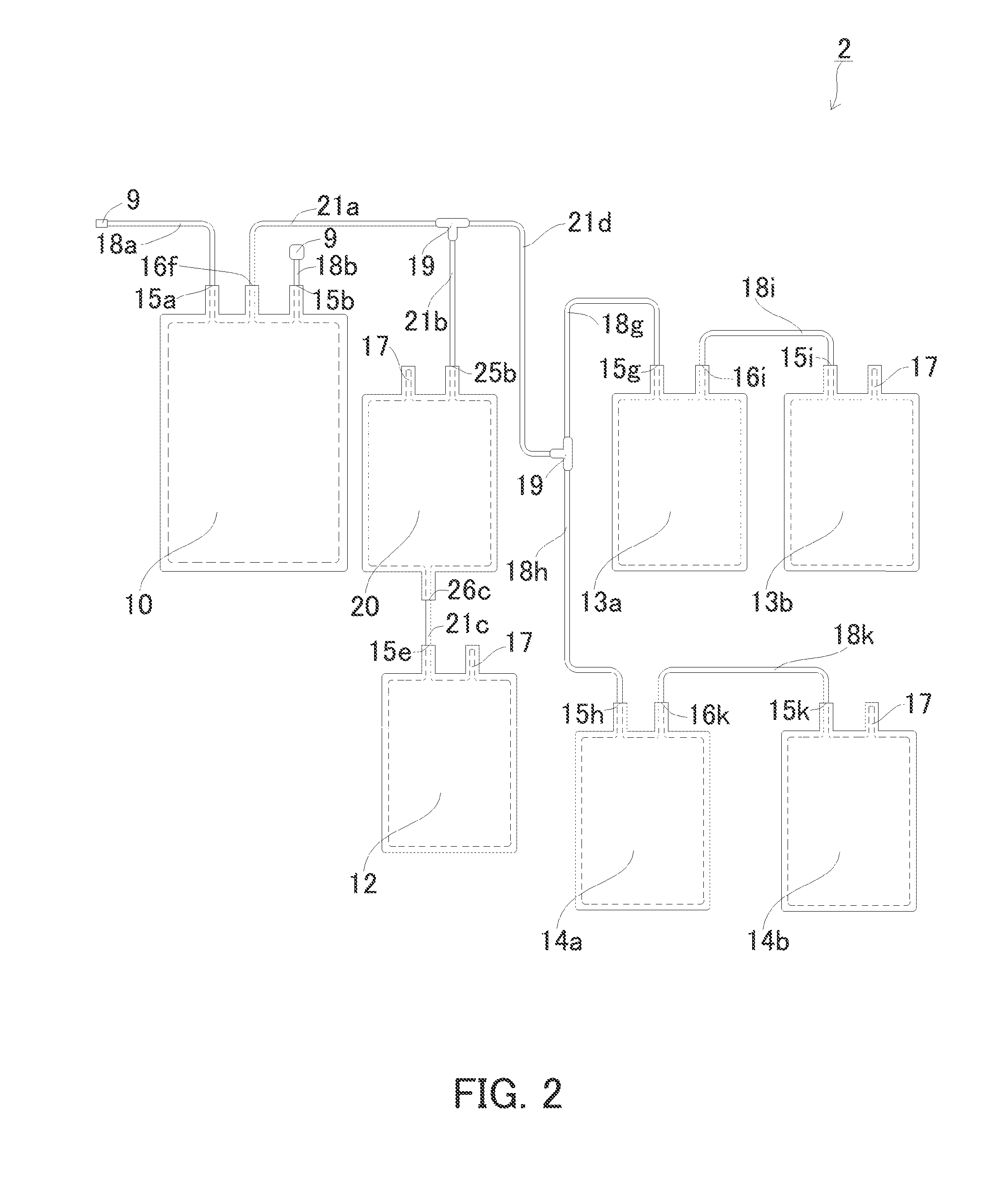
[0162]Next, an example of the second cord blood component preparation device according to the present invention will be described with reference to FIG. 2. In FIG. 2, elements the same as those in FIG. 1 are marked with the same reference numerals. The second cord blood component preparation device is the same as the first cord blood component preparation device, unless otherwise stated. Unless otherwise stated, the present invention is not limited thereto.
[0163]FIG. 2 is a plan view schematically showing the cord blood component preparation device of the present embodiment. The cord blood component preparation device 2 includes: a storage unit 10 for storing cord blood; a cell-containing plasma accommodation unit 20 for accommodating hematopoietic stem cell-containing plasma obtained after removing erythrocytes; a hematopoietic stem cell accommodation unit 12 for accommodating hematopoietic stem cells; a first plasma accommodation unit 13a and a second plasma accommodation unit 14a...
third embodiment
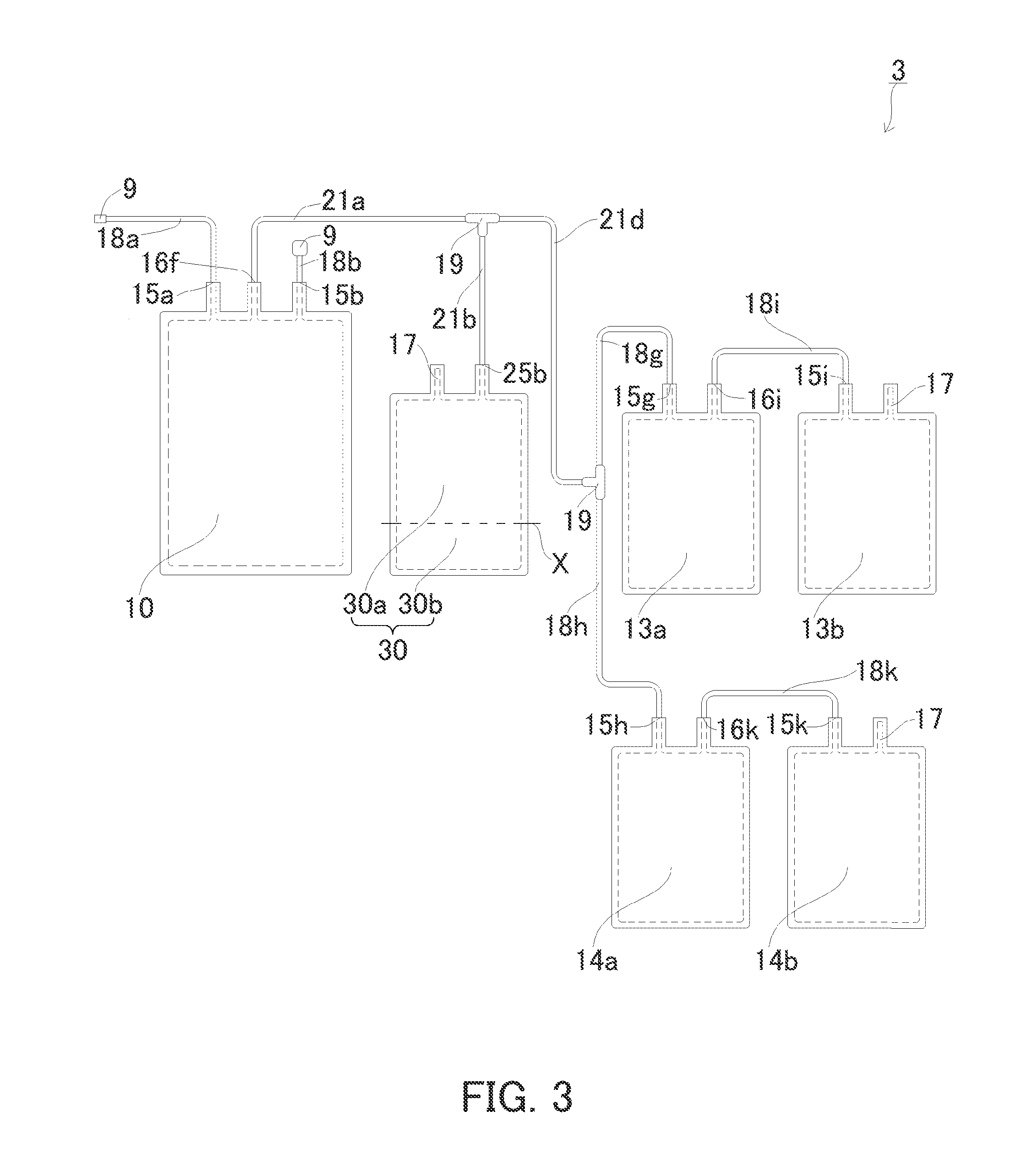
[0170]Next, an example of the third cord blood component preparation device according to the present invention will be described with reference to FIG. 3. In FIG. 3, elements the same as those in FIGS. 1 and 2 are marked with the same reference numerals. The third cord blood component preparation device is the same as the first and second cord blood component preparation devices, unless otherwise stated. Unless otherwise stated, the present invention is not limited thereto.
[0171]FIG. 3 is a plan view schematically showing the cord blood component preparation device of the present embodiment. The cord blood component preparation device 3 is the same as the second cord blood component preparation device described above, except that it includes, instead of the cell-containing plasma accommodation unit 20 and the hematopoietic stem cell accommodation unit 12 shown in FIG. 2, a dividable cell-containing plasma accommodation unit 30. The cell-containing plasma accommodation unit 30 is con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com