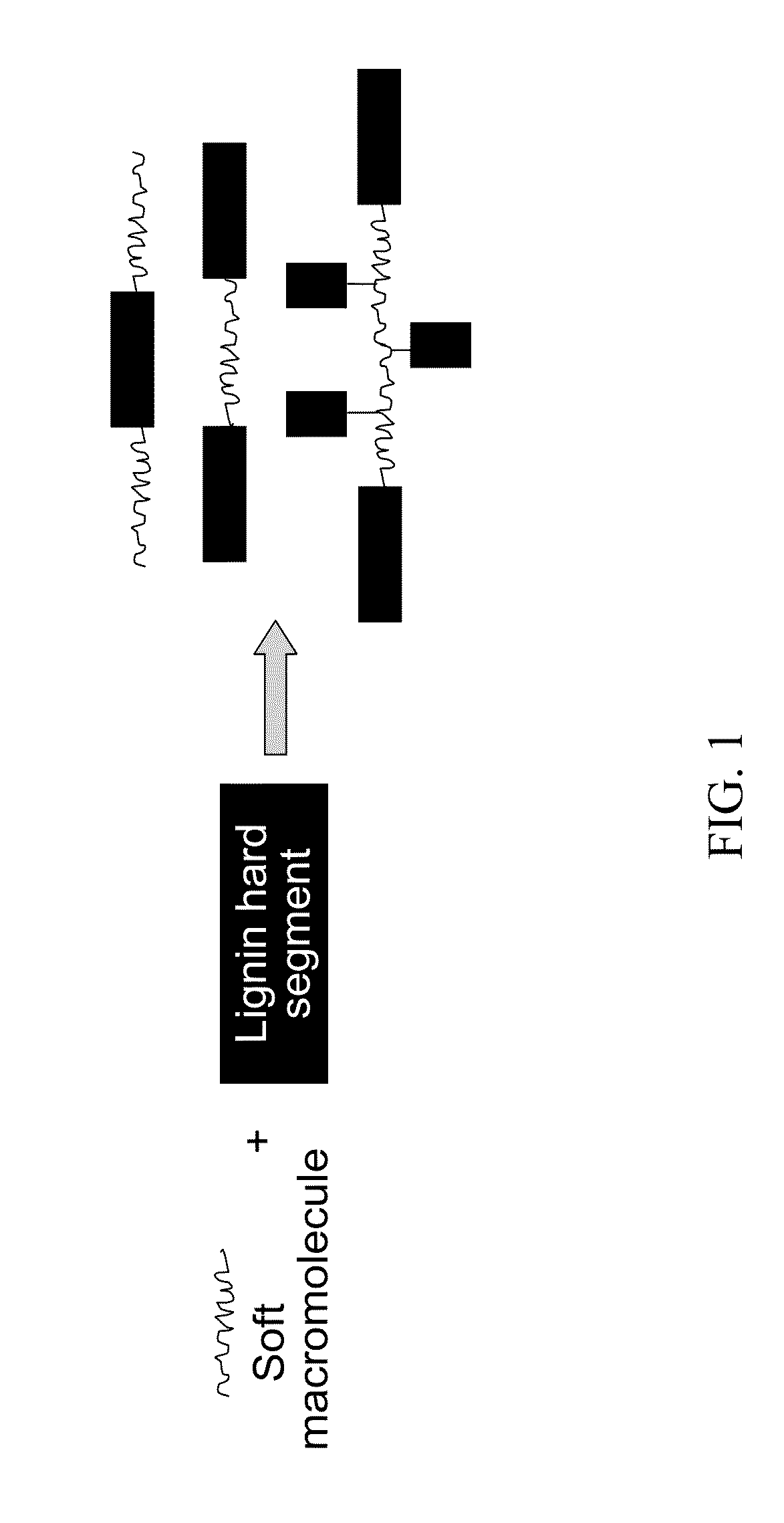
Lignin-derived thermoplastic co-polymers and methods of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Controlled Crosslinking of Lignin
[0059]The process for crosslinking lignin described in this example can be generally summarized by the following reaction scheme:
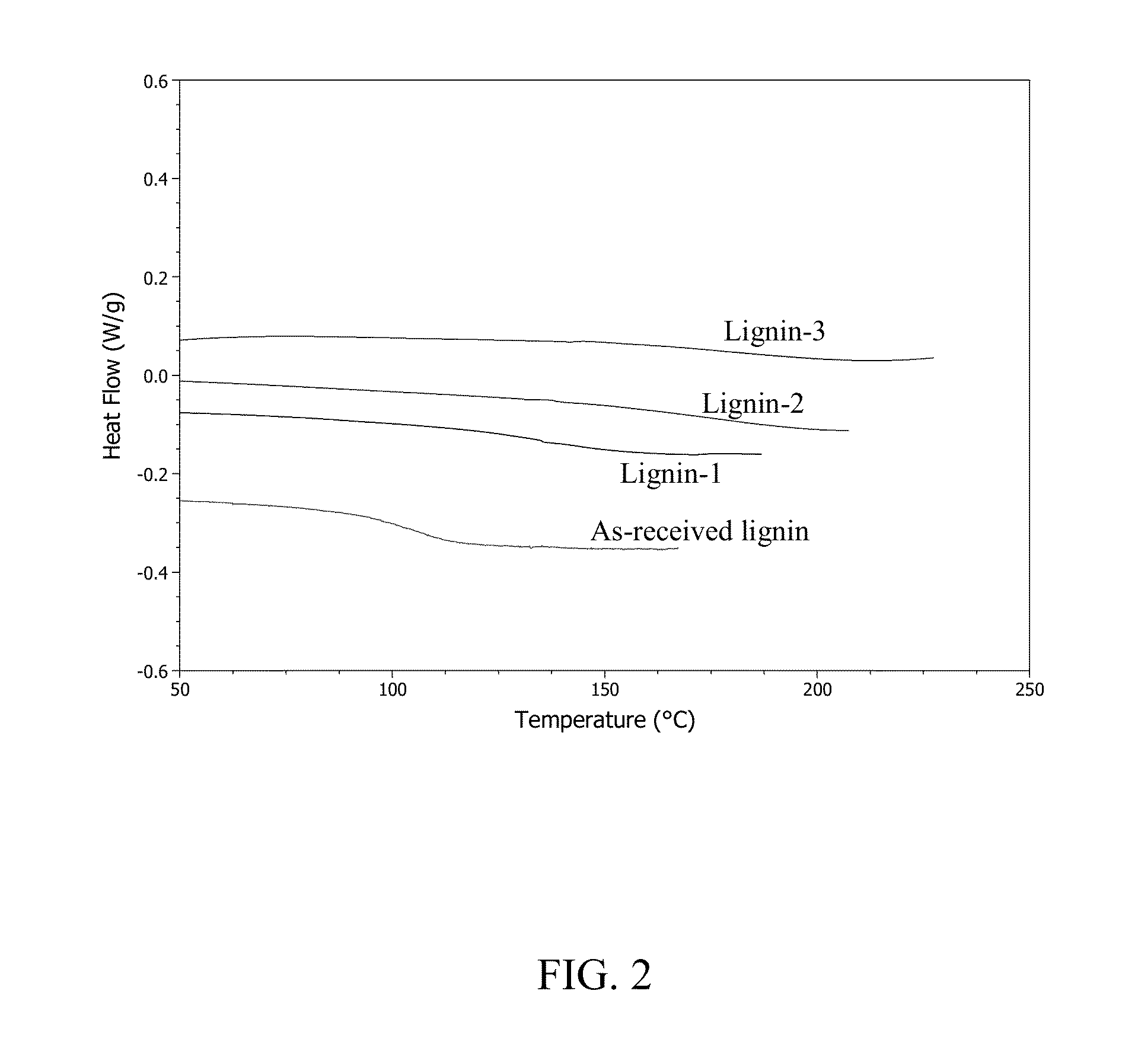
[0060]A 1-L round-bottomed flask was equipped with a large magnetic stir bar and placed in a heating mantle. 100 g of organosolv lignin (as received, not dried, having a MW of 1840 g / mol, polydispersity index (ratio of weight average to number average molecular weights) of 122, and Tg of 108° C.) were added to the flask. Lignin was dissolved using 200 mL NaOH solution (pH>14) and 300 mL of millipure water, for a final concentration of 16 wt % solids. Once the lignin appeared fully dissolved, the flask was fitted with an addition funnel. 120 mL of formalin solution (10 wt % in buffered solution) were added dropwise to the lignin solution with stirring, at 80° C. After two hours, an additional 120 mL of formalin (10 wt % in buffer solution) were added to the reaction. Reaction continued for four additional hours (six hours to...
example 2
Copolymerization Reactions of Crosslinked Lignin
[0063]I. Copolymerization Reaction of Crosslinked Lignin with Carboxy-Functionalized Polybutadiene
[0064]The process for copolymerizing crosslinked lignin with carboxy-functionalized polybutadiene described in this example can be generally summarized by the following reaction scheme. The long curved lines shown in the final product represent carboxy-functionalized polybutadiene that has been attached to the crosslinked lignin.
[0065]As-received lignin was reacted with dicarboxy-terminated polybutadiene soft segment (Mn 4,200 g / mol) in the presence of formalin at 1:1 F / L ratio with KOH in 1,4-dioxane solvent (with KOH concentration of 2.4 mmol / 40 mL solvent) for 24 hours at 100° C. Different amounts of lignin were loaded to obtain 9, 13, 17, and 22 wt % lignin, respectively, in the feed compositions. All the resulting lignin-polybutadiene copolymers were isolated via precipitation into methanol and dried under reduced pressure at 50° C. f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com