Polymorphisims in the human cyp2d6 gene promoter region and their use in diagnostic and therapeutic applications
a technology of cyp2d6 and promoter region, which is applied in the direction of enzymology, drug composition, cardiovascular disorder, etc., can solve the problems of individual um phenotype at risk of therapeutic failure, adverse side effects, etc., and achieves improved drug tolerance, reduced overhead, and improved health care
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0059]Genomic samples, isolated by standard techniques from human blood samples were obtained from healthy volunteers under consideration of all legal, ethical and medical requirement of the local ethics committee. Blood samples from >50 individuals were obtained and processed by ion exchange chromatography methods (Qiagen) to isolate DNA.
1. Description of Methods:
[0060]DNA Samples and PCR Conditions:
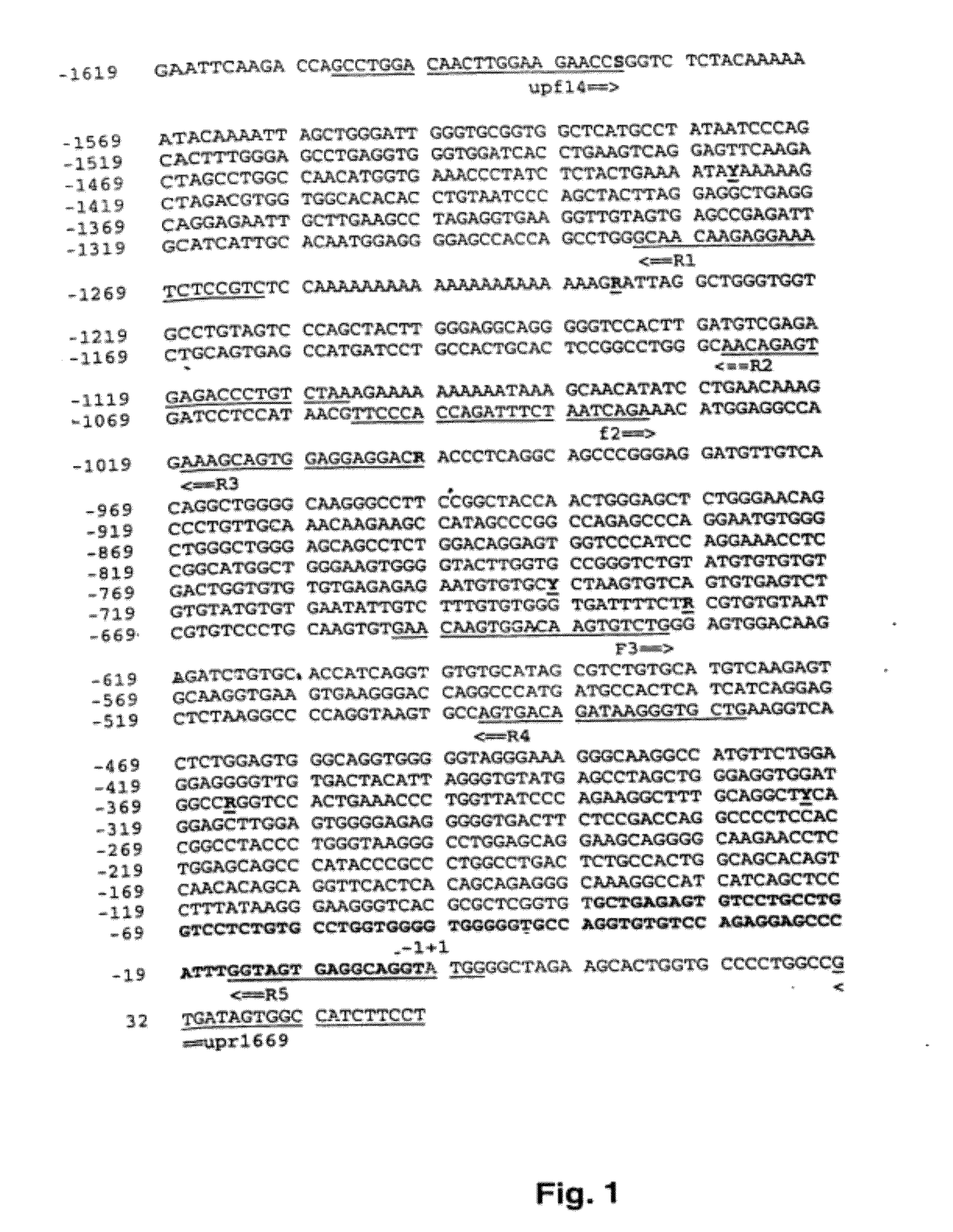
[0061]Leucocyte DNA from Caucasian individuals was isolated from blood samples by standard methods. All indiciduals included in this study had been previously phenotyped with sparteine and genotyped for the CYP2D6-polymorphism using published methods (StUven et al., 1996; Griese et al., 1998). To investigate the promoter region of the CYP2D6 gene, genomic DNA was amplified using a pair of primers upf14 and upr1669, designed to specifically amplify a 1656 by fragment which contains almost the entire known 5′-flanking sequence (FIG. 1, Table 1). Aliquots of each PCR product were subjected...
example 2
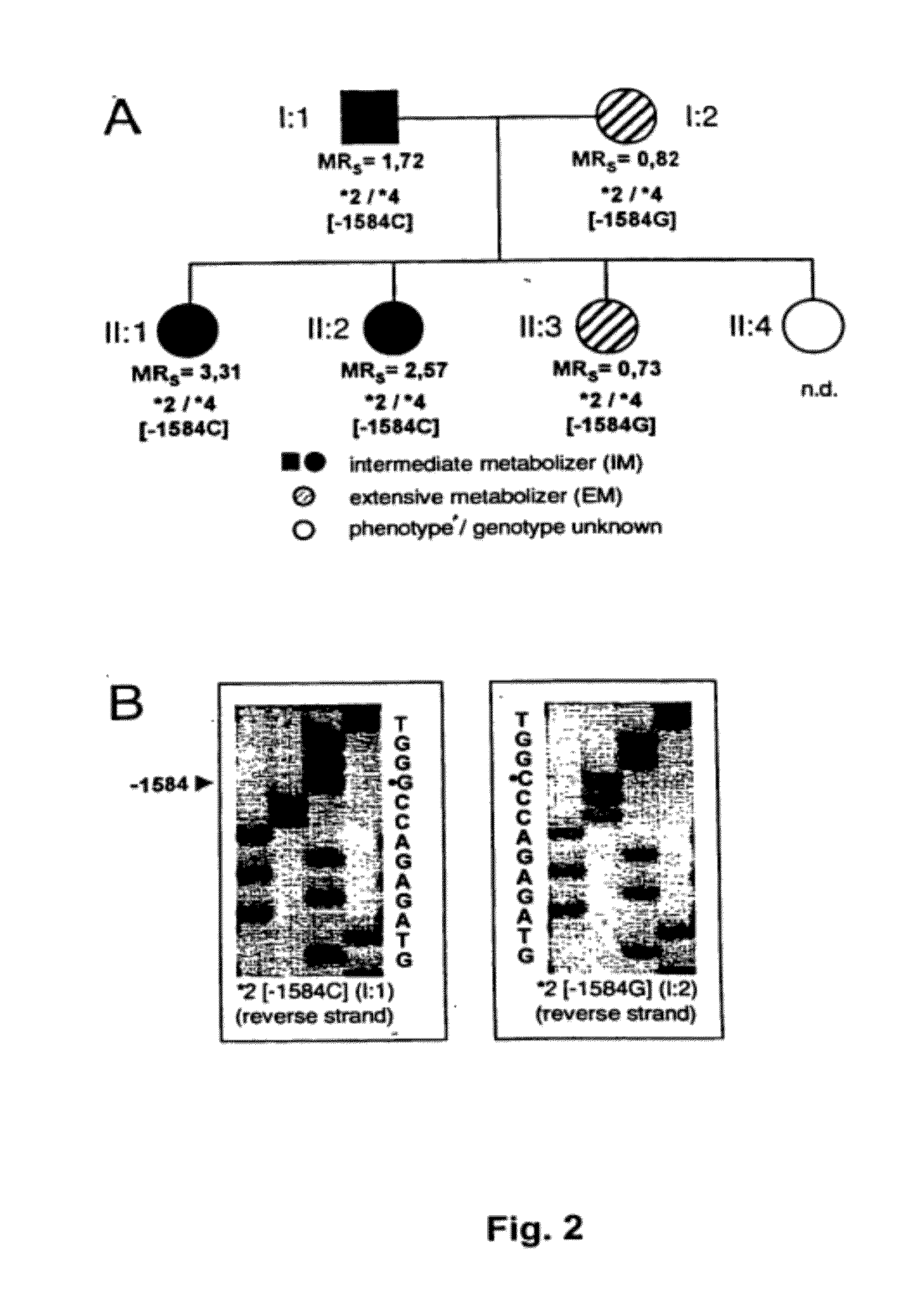
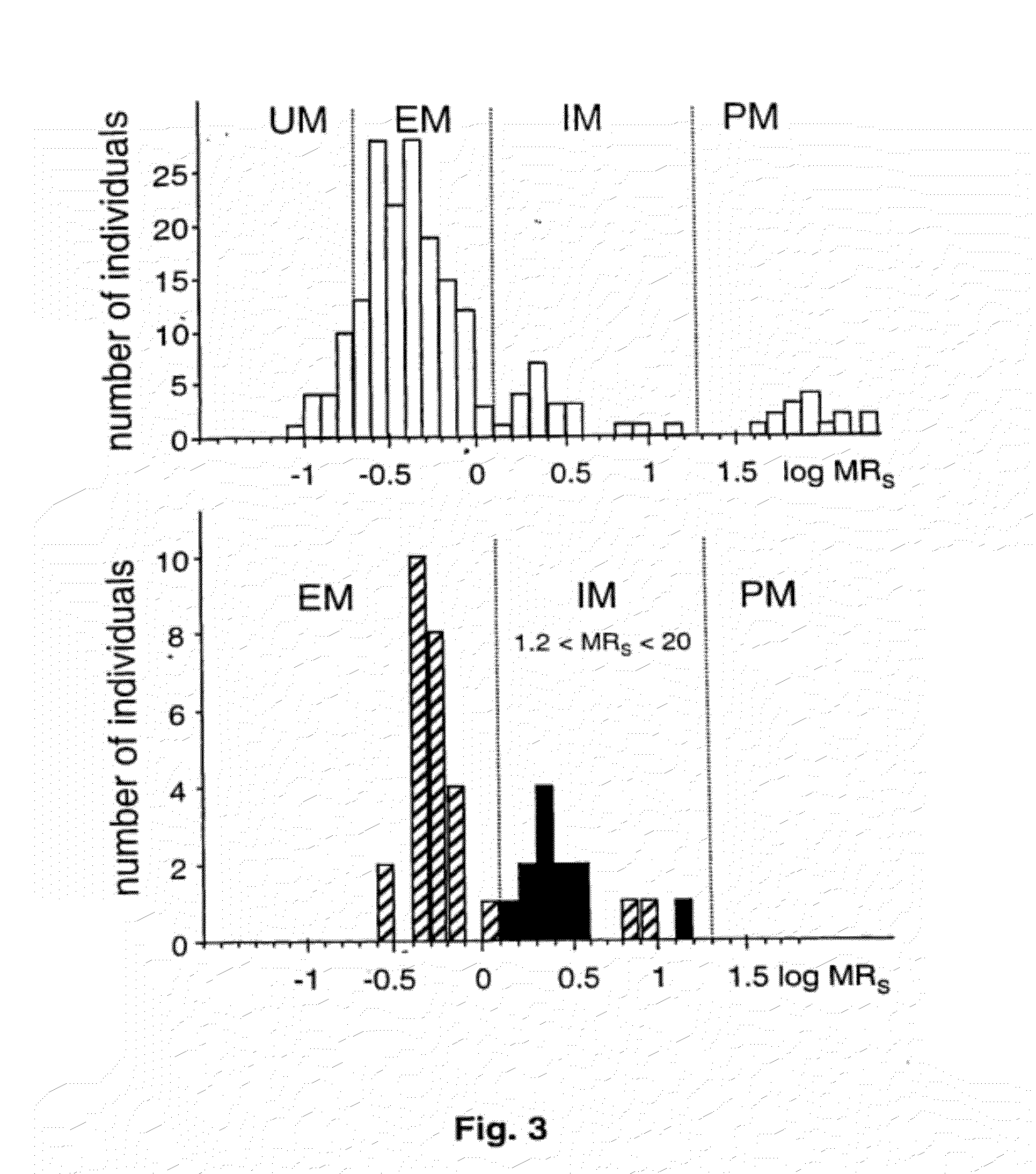
[0072]CYP2D6 was quantitated immunologically and enzymatically in liver biopsies from 76 individuals with known sparteine oxidation phenotype. All important known alleles including the −1584 C / G promoter polymorphism were determined.
1. Description of Methods:
[0073]Patients and Liver Samples. Liver tissue was obtained as wedge biopsy specimens from patients undergoing cholecystectomy between 1983 and 1986 and stored at −80° C. All patients included here had been phenotyped with respect to their sparteine metabolizer phenotype before laparotomy (Osikowska-Evers et al., 1987).
[0074]CYP2D6 Genotypinq. CYP2D6 alleles were assigned based on the determination of the appropriate key mutations using established PCR assays for alleles *2, *3, *4, *5, *6, *7, *8, *9, and *10 (Steven et al., 1996; Griese et al., 1998). The C / G mutation was determined using a novel real-time PCR method (Zanger et al., 2001).
[0075]Preparation of DNA and of Liver Microsomes. Genomic DNA was isolated from a fractio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| nucleic acid | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com