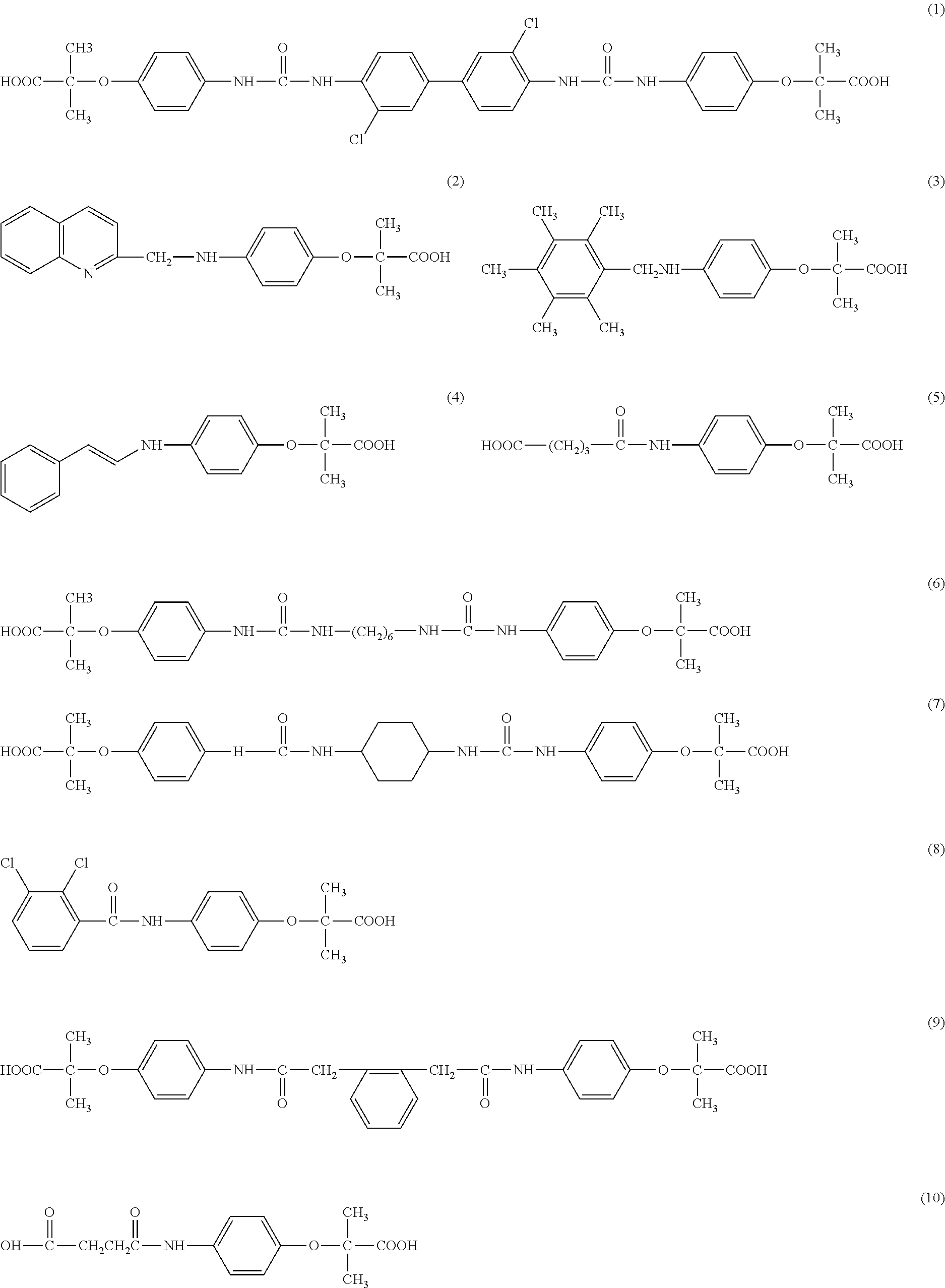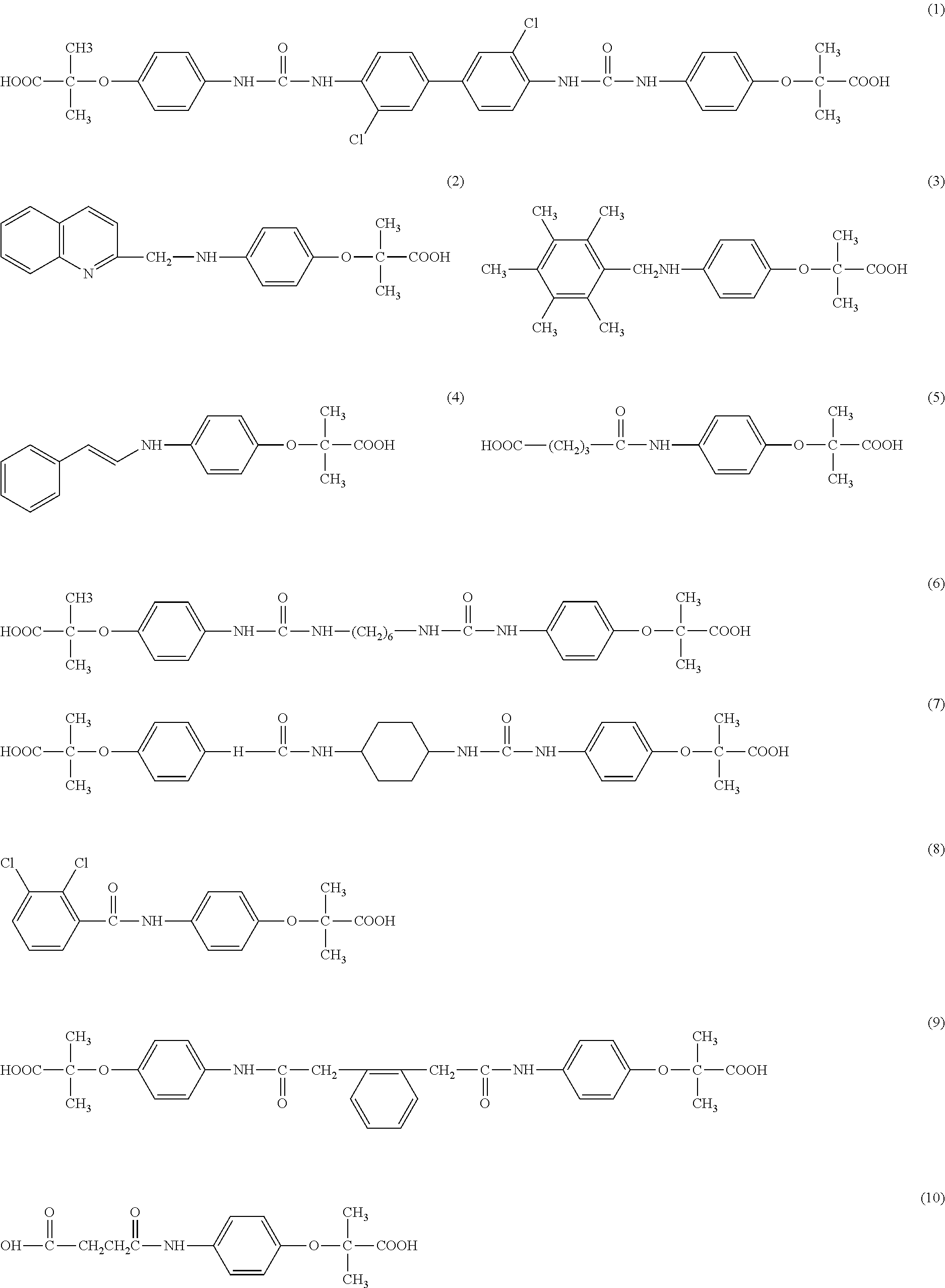Novel phenoxyisobutyric acid compounds and methods for synthesis
a technology of phenoxyisobutyric acid and phenoxyisobutyric acid, which is applied in the direction of peptide/protein ingredients, application, metabolism disorders, etc., and can solve the problems of severe structural and functional changes in protein/protein and protein/cell interaction in the vascular wall, severe consequences on affected organs, and inability to fully understand the mechanisms of hyperglycemia-induced tissue damage in diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1 (
Compound 1)
[0045]3,3′-dichloro-1,1′-diphenyl-4,4′-diureidophenoxyisobutyric Acid
[0046]A mixture of 1.95 g (0.01 mole) of 4-aminophenoxyisobutyric acid and 3,3′-dichloro-1,1′-diphenyl-4,4′-diisocyante in 30 ml of cooled tetrahydrofuran (THF) and 0.4 g of NaOH are combined and stirred at room temperature for 24 hours. The THF is evaporated by blowing air. Excess water is added and to the resulting brown solution, about 1.0 g of dithionite is added and the precipitate is separated by filtration (slow) and washed twice with hot water. The filtrate is acidified with concentrated acetic acid and filtered The white powdery material is washed with cold water and dried with dithionite, filtered and acidified with acetic acid The white precipitate is filtered and dried giving 1.6 g (dry) yield 43%. The structure is set forth as structure 1.C34H32Cl2N4O8; mw 695
example 2 (
Compound 2)
[0047]Quinoline-2-(methyleneaminophenoxyisobutyric) Acid
[0048]A mixture of 1.95 g (0.01 mole) of 4-aminoiphenoxyisobutyric acid, 2.28 g (0.01 mole) of 2-chloromethylquinoline hydrochloride; 3 g of potassium carbonate,40 ml of water and 29 ml of isopropanol is refluxed and stirred for 24 hours and a dark brown solution with about 1 g of product is obtained. The product is worked up by adding sodium carbonate. Dithionite is added and the mixture is acidified with acetic acid and a brown powder is obtained. The structure is set forth as structure 2..C20H20N2O3; mw 336
example 3 (
Compound 3)
[0049]2,3,4,5,6-(pentamethylbenzyl-4-aminophenoxyisobutyric) Acid
[0050]A mixture of 1.25g (0.005 mole) of 3,6-bis(chloromethyl amine); 1.9 g. (0.01 mole) 4-aminophenoxyisobutyric acid, 2.0 g (0.01 mole) potassium carbonate and 25 ml of a 50:50 mxture of water and isopropanol is stirred for 24 hours. The mixture is treated as in Example 1 giving a solid with a melting point of 178-184° C.The structure is shown as structure 3 C22H29N1O3;mw 455
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com