Vortex-induced silk fibroin gelation for encapsulation and delivery
a technology of silk fibroin and encapsulation, which is applied in the direction of antibody medical ingredients, peptide/protein ingredients, drug compositions, etc., can solve the problems of limiting cell viability and altering cell or bioactive molecule function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Aqueous Silk Fibroin Solutions
[0111]Silk fibroin aqueous solutions were prepared as previously described in the literature. See Sofia et al., 54 J. Biomed. Mater. Res. 139-48 (2001). Briefly, Bombyx mori cocoons were boiled for 40 min in an aqueous solution of 0.02 M NaCo3 and then rinsed thoroughly with deionized water. After overnight drying, the extracted silk fibroin was dissolved in an aqueous solution of 9.3 M LiBr at 60° C. overnight. The resulting solution was dialyzed against deionized water using Slide-A-Lyzer dialysis cassettes (MWCO 3,500, Pierce, Thermo Scientific, Waltham, Mass.) for two days to remove the residual salt. The final concentration of the silk fibroin was approximately 5.3 wt %. Lower concentration silk solutions were prepared by diluting the 5.3 wt % stock solution with deionized water. Additionally, the silk fibroin solution may be concentrated, for example, to about 30% (w / v). Briefly, the silk fibroin solution with a lower concentration ...
example 2
Vortex-Induced Hydrogelation
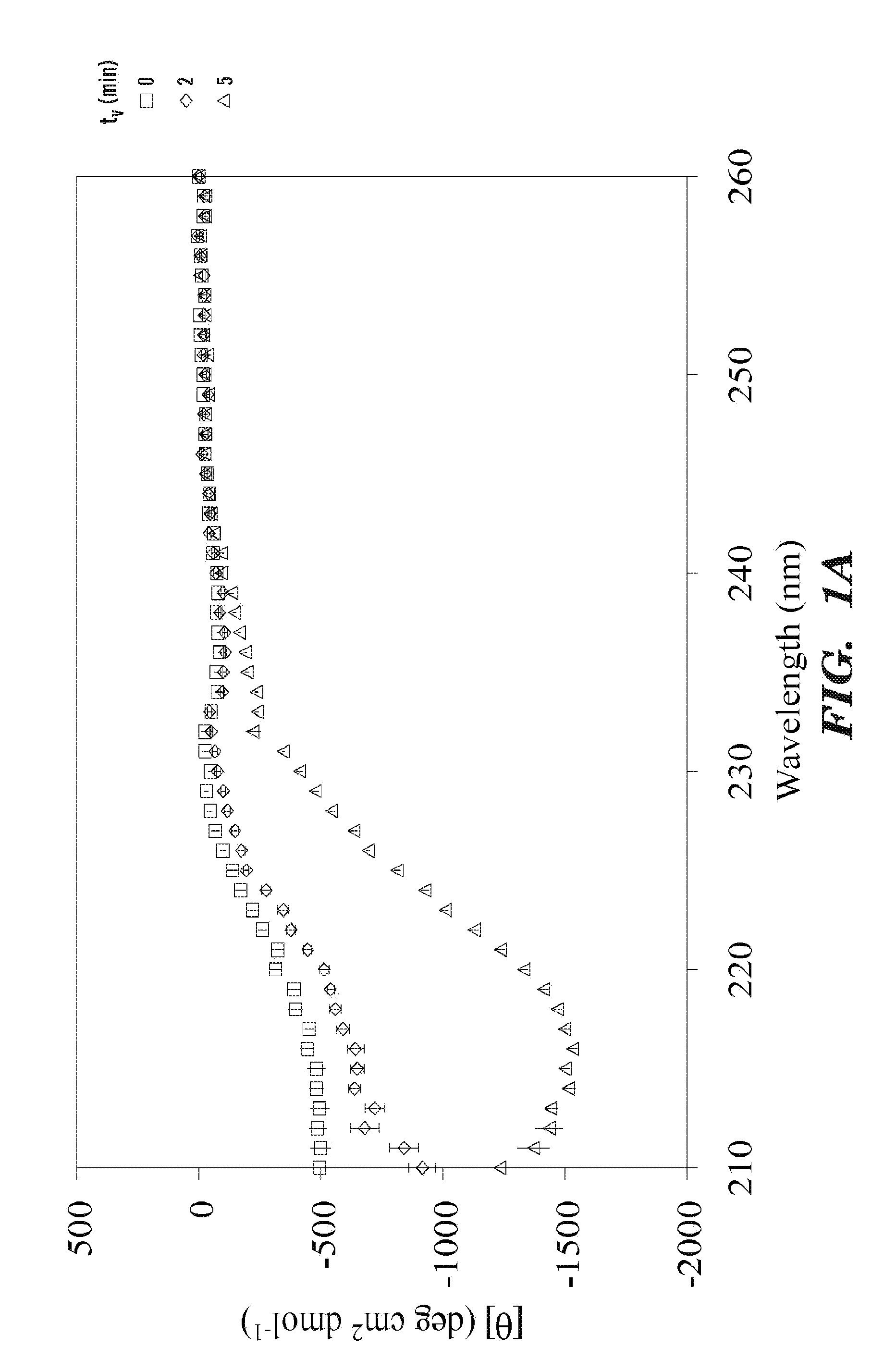
[0113]A 1 ml aliquot of silk solution was equilibrated at 25° C. in a vial kept in a water bath for 10 min and mixed for predetermined times at 3,200 rpm using a vortexer (Fisher Scientific, Pittsburg, Pa.) to induce silk self-assembly and hydrogelation. Such prepared silk hydrogelation was characterized by CD and rheology experiment. Increasing the vortex time increased the solution turbidity and eventually bulk phase separation of a white and solid-like material was observed, especially at lower protein concentrations. Both CD and rheology data were collected from turbid solutions after removal of the solid phase.
example 3
Circular Dichroism Spectroscopy
[0114]Circular dichroism (CD) spectra were collected using an Aviv Model 410 CD spectrometer (Aviv Biomedical, Inc., Lakewood, N.J.). After vortexing, aqueous silk solutions were immediately loaded in a 0.1 mm quartz cell within a temperature controlled cell holder. CD wavelength scans between 210 nm and 260 nm or time sweeps at 216 nm were collected at 25° C. Due to the high silk concentrations, the high dynode voltages below 210 nm lead to erroneous data. The CD spectrum of water collected immediately before each measurement was used for background correction.
[0115]The mean residual ellipticity was calculated from
[θ]=θ·M10·c·l·n(deg·cm2dmol)
where θ is the measured ellipticity (deg), M is the mean molecular mass (g / mol), c is the protein concentration (g / cm3), 1 is the path length (cm), and n is the number of residues. M and n for B. mori heavy chain were taken as 391,563 g / mol and 5263, respectively (Zhou et al, 28 Nucleic Acids Res. 2413-19 (2000))....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com