Targeting an hiv-1 nef-host cell kinase complex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Recombinant Protein Expression and Purification
[0127]Hck-YEEI and Nef were expressed in Sf9 insect cells and purified as described, for example, by Trible, 2006, Id.
example 2
In Vitro Kinase Assay and Chemical Library Screening
[0128]Protein-tyrosine kinase assays were performed in 384-well plates using the Z′-LYTE™ kinase assay system and “Tyr 2 peptide” substrate (Invitrogen, Carlsbad, Calif.) as described by Trible et al. (Id.) Chemical libraries were purchased from ChemDiv, Inc. (San Diego, Calif.) and include a kinase-directed library (2500 compounds), a phosphatase directed library (2500 compounds) and a diversity set (5040 compounds). Library screens were conducted 384-well plates in a final volume of 10 μl per well. Compounds were added to each well (10 μM final), followed by a preformed complex of Hck-YEEI (10 ng / well) and Nef (1:20 molar ratio) plus the substrate peptide (2 μM). Reactions were initiated by the addition of ATP (50 μM final) and incubated at room temperature for 35 minutes. Reactions were developed and terminated per manufacturer's protocol, and with fluorescence ratios calculated as described (Trible et al., Id.)
example 3
HIV Replication Assay
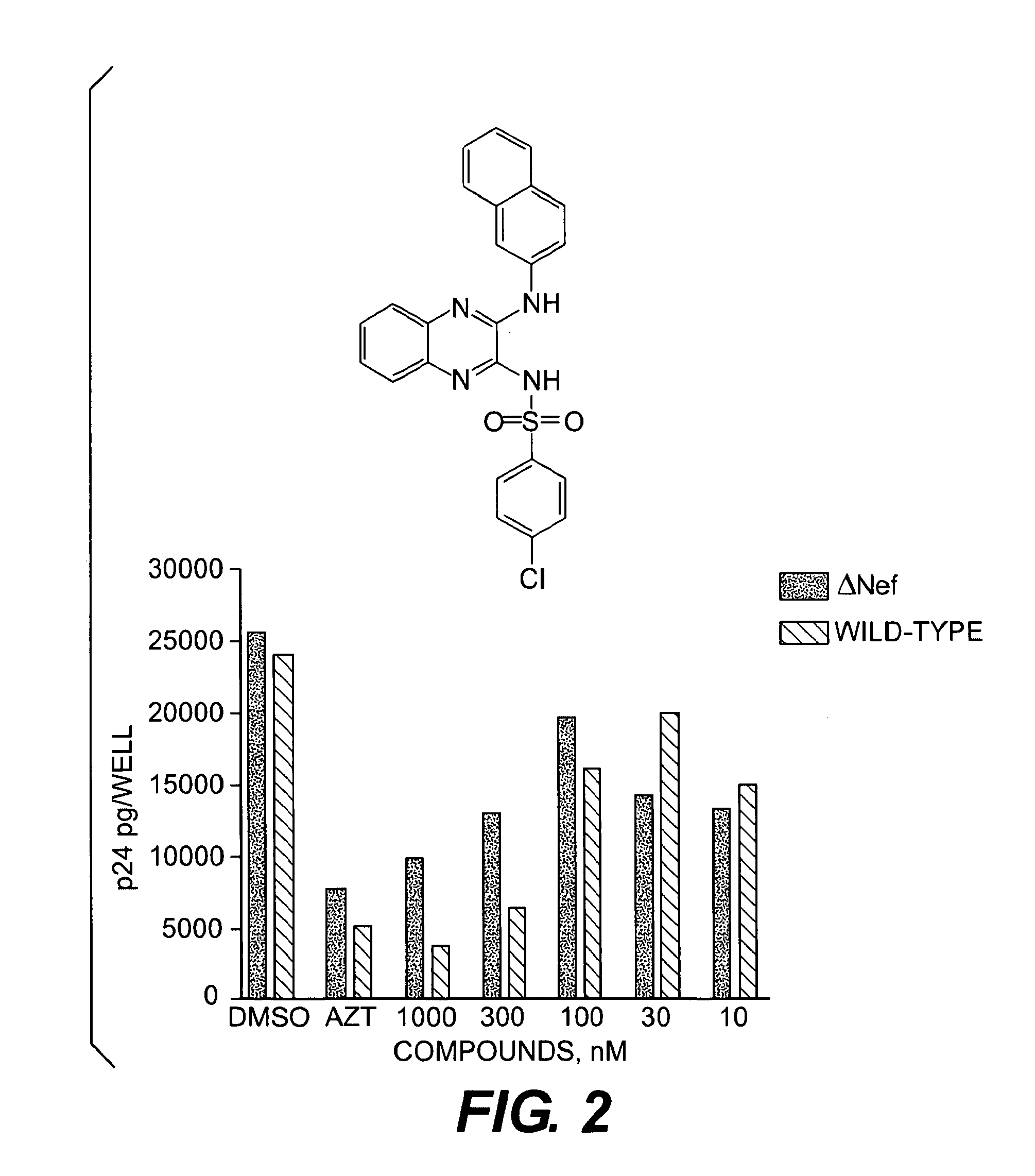
[0129]HIV-1 replication assays were conducted using HIV-1 strain NL4-3, a strain that is very similar, as known to those of skill in the art, in sequence to the SF2 allele used in the yeast assays and that strongly activates Hck-YEEI. Virus stocks were prepared by transfection of the recombinant viral genome into 293T cells. Viral replication was monitored in the U87MG astroglioma cell line expressing CD4 and CXCR4 (Salvatori, F. & Scarlatti, G., 2001, AIDS Res. Hum. Retroviruses 17: 925-35; Trkola, A. et al., 1998, J. Virol. 782:1876-1885). Viral replication was monitored by measuring p24 protein levels in the culture supernatant 4 days after infection by standard ELISA-based techniques. Test compounds were added to the culture 30 min prior to infection with HIV, and DMSO was used as the carrier solvent at a final concentration of 0.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com