Extracellular targeted drug conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Linker Ready Therapeutic Agents and Biotin
[0288]To show that illustrative compounds of the invention are useful at targeting and killing tumor cells, therapeutic agents with activity toward the Na,K-ATPase were synthesized and then coupled to Na,K-ATPase specific antibodies via non-cleavable linkers. The agents were synthesized with active groups which could be coupled to non-cleavable linkers. The agents can also be used as controls to test agent activity when not coupled to antibodies via the linkers.
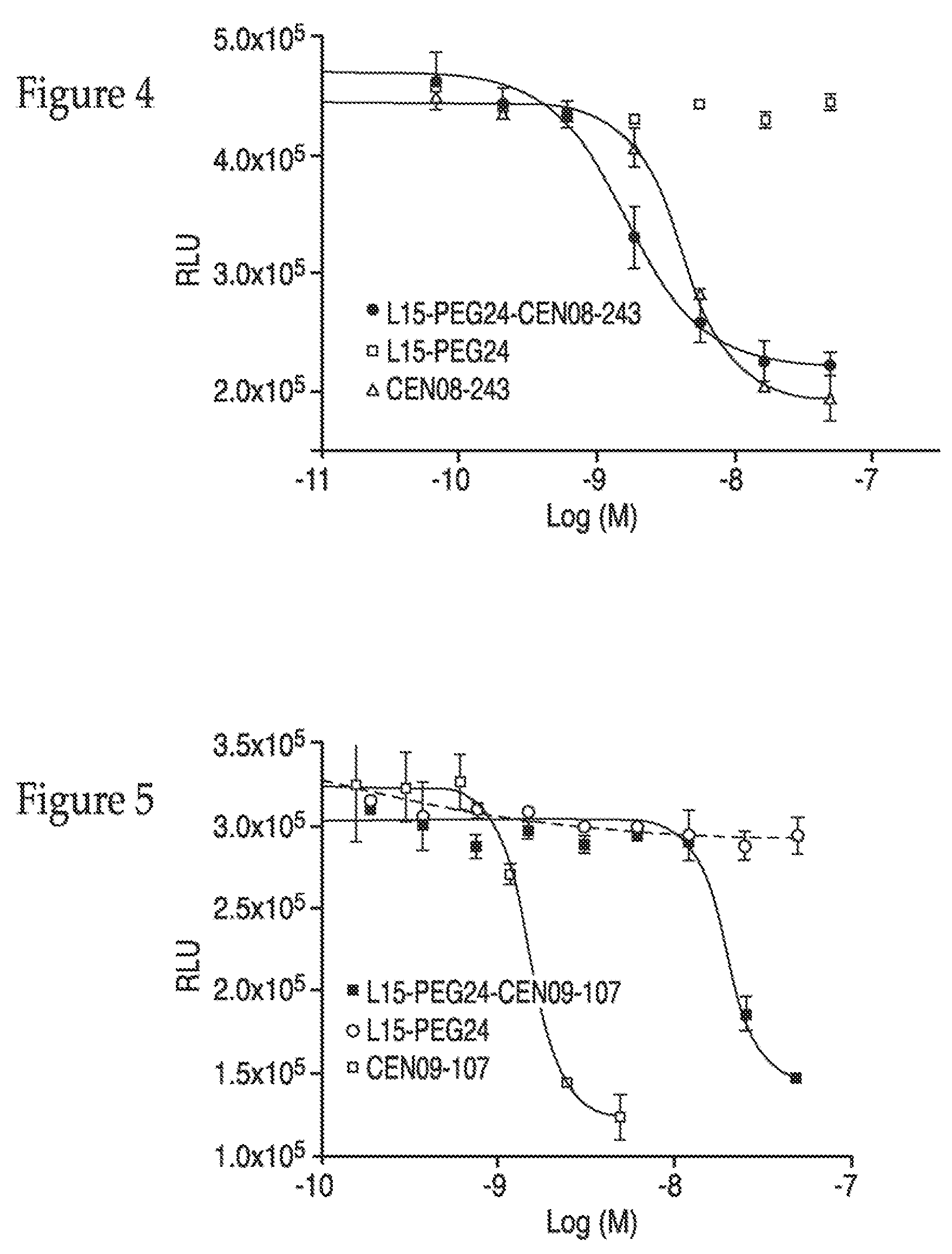
[0289]Glycinyl hydrazone of scillarenone (CEN09-104) was prepared as follows and used in Example 3.
[(tert-Butyloxycarbonyl)amino]acetyl-hydrazine. N-Boc-glycine methyl ester (2.12 g, 10.87 mmol) and hydrazine hydrate (3.2 g, 54.34 mmol) were stirred for 2 hours at reflux. The solvent was removed under reduced pressure and the crude was purified by flash chromatography (CH2Cl2 / MeOH, 90:10), the NMR data agreed with literature. (Borg, S.; Estenne-Bouhtou, G.; J. Org. Chem. ...
example 2
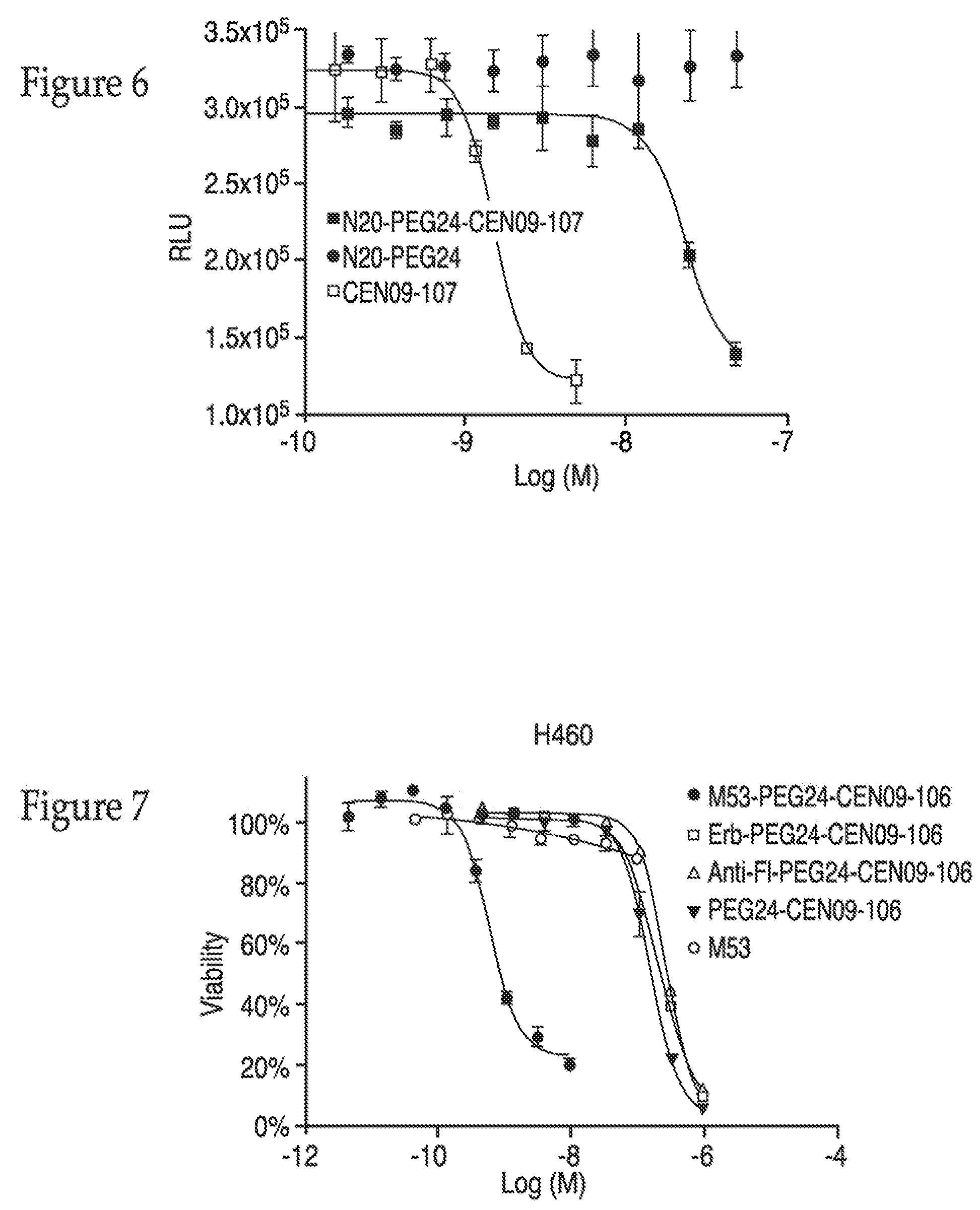
Mapping of the Epitope to which Antibody M53 Binds
[0309]To better understand the recognition of antibodies used for the illustrative compounds of the invention, epitope mapping was conducted. If the genetic sequence or protein sequence is known or determined for the EDC's antibody's target, epitope mapping may allow the pre-use determination of EDC activity. Therefore, where not previously determined, linear epitope recognition of the antibodies used in examples 3, 4, 5, 6, 7 and 8, was determined. Overlapping peptide sequences were synthesized that represent positions 24 through 145 of the extracellular domain of human FXYD5, each peptide was synthesized with a cysteine at its amino terminus to facilitate conjugation to maleimide activated BSA (cat. number: 77116, Pierce Biotechnology). Peptides were coupled to BSA per the manufactures protocol, and any unreacted maleimide groups of the BSA were capped by the addition of L-cysteine. ELISA plates (cat. number: 9017, Corning) were co...
example 3
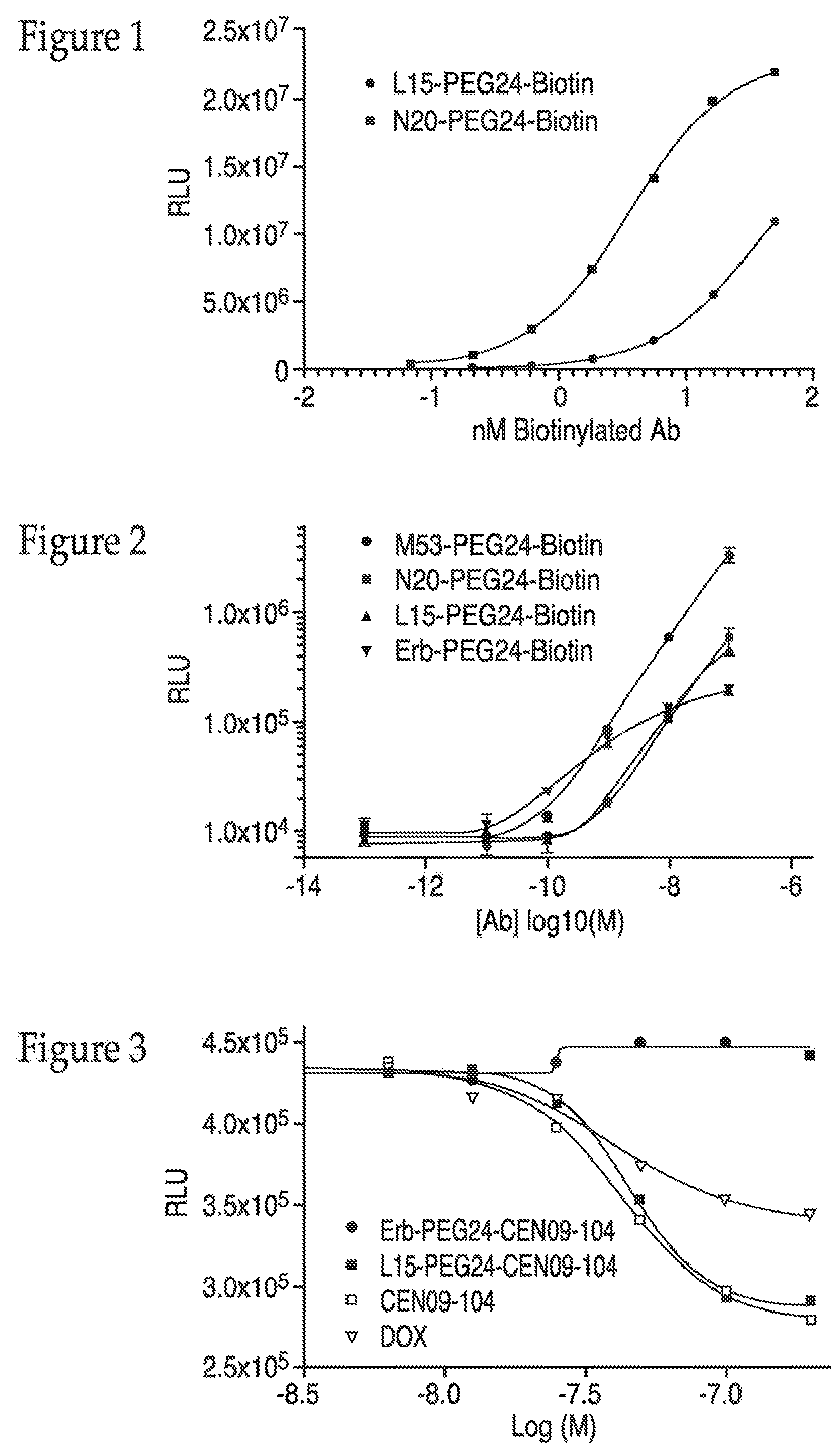
Production and Testing of Antibody-Linker-Biotin Conjugates
[0314]To demonstrate that the agent linking chemistry performs and does not interfere with antibody binding, biotin was first coupled to antibodies via the same conditions and non-cleavable bifunctional PEG24 linker employed to couple the agents used in Example 4 to show EDC activity. First, linker SM(PEG)24 [Thermo Scientific product number 22114] was attached to EZ-Link Amine-PEG2-Biotin [Thermo Scientific—product number 21346] followed by coupling to BME reduced antibodies. The antibody-biotin conjugates were then tested for binding to cells and peptide of sequence 24-39 of Seq ID 1.
[0315]Coupling linker to biotin. 1.9 mg of EZ-Link Amine-PEG2-Biotin were dissolved in 100 μl of DMSO to obtain a 50 mM solution. 2 μl of a 250 mM stock of linker SM(PEG)24 was added to 98 μl of DMSO to obtain a 5 mM solution. 50 μl of the 5 mM linker solution were added to 50 μl of the 50 mM Biotin-NH2 solution and di-isopropyl ethylamine (fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com