Method For Replacing Native Valve Function Of A Diseased Aortic Valve
a technology of native valve function and aortic valve, which is applied in the field of replacing native valve function of diseased aortic valve, can solve the problems of uncontrollable bleeding, low success rate of aortic valve delivery system, and low progress in the development of safer and less invasive valve delivery systems, so as to reduce the risk of embolic debris, pass easily and reliably, and avoid uncontrollable bleeding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
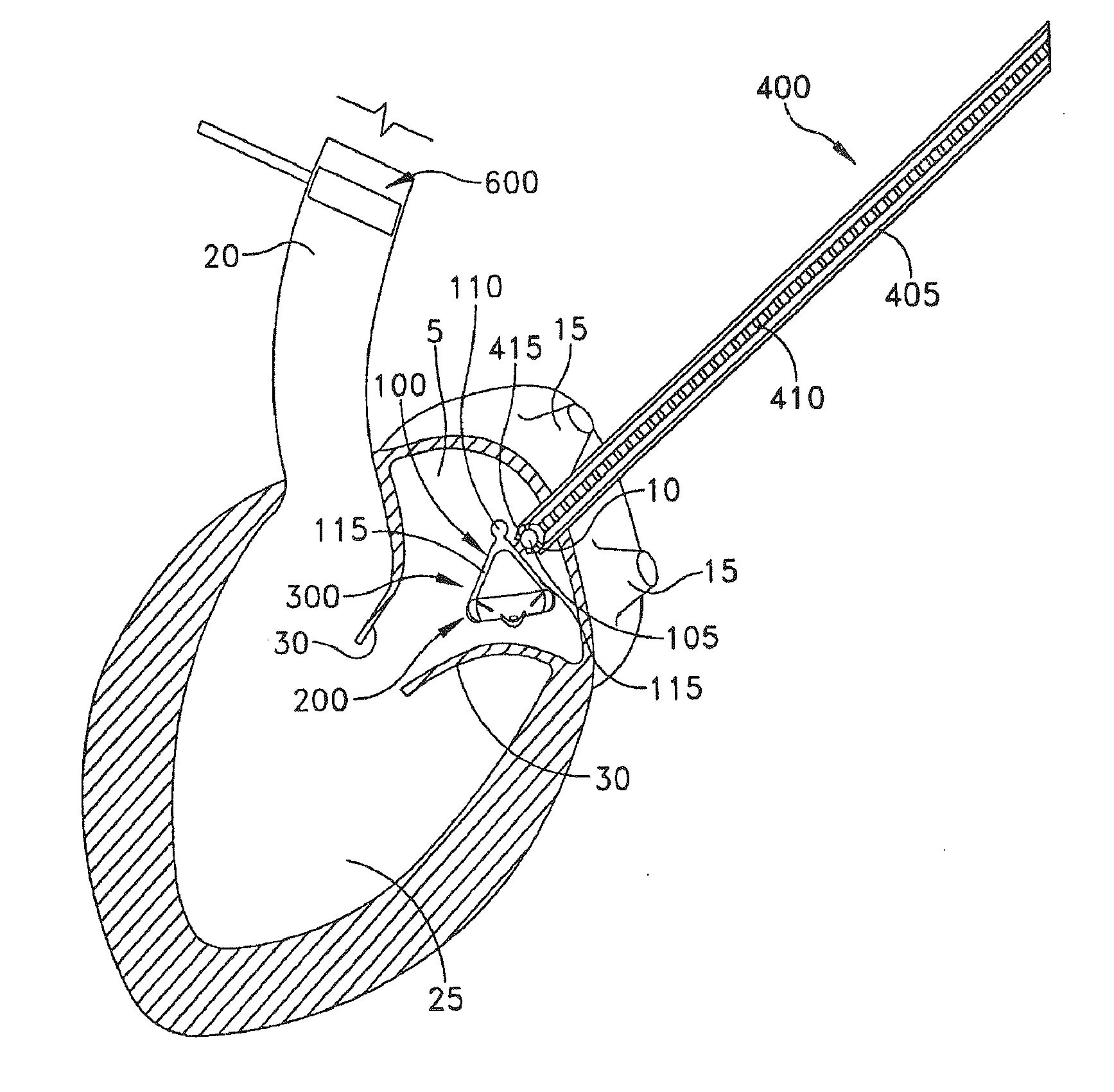
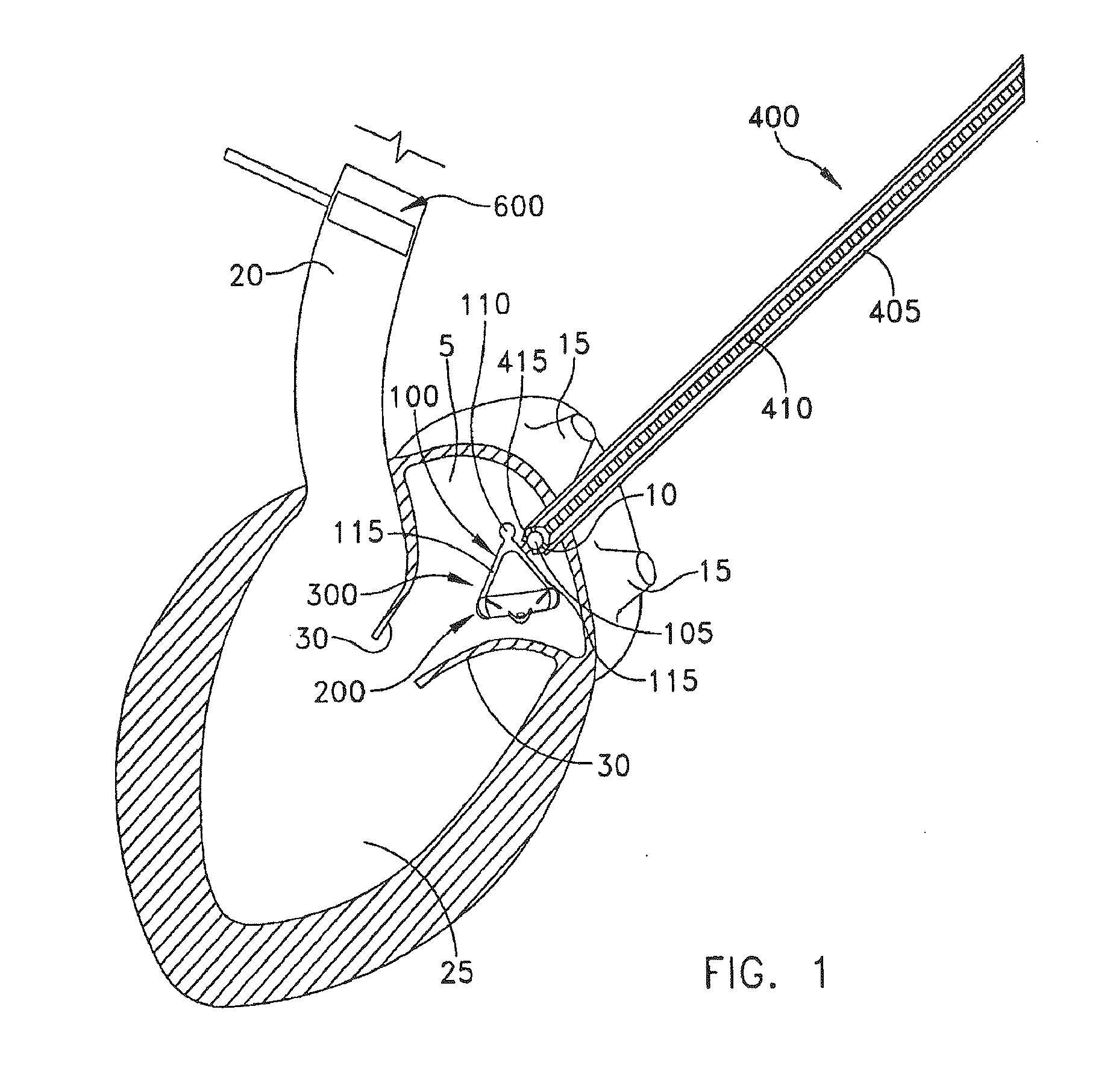
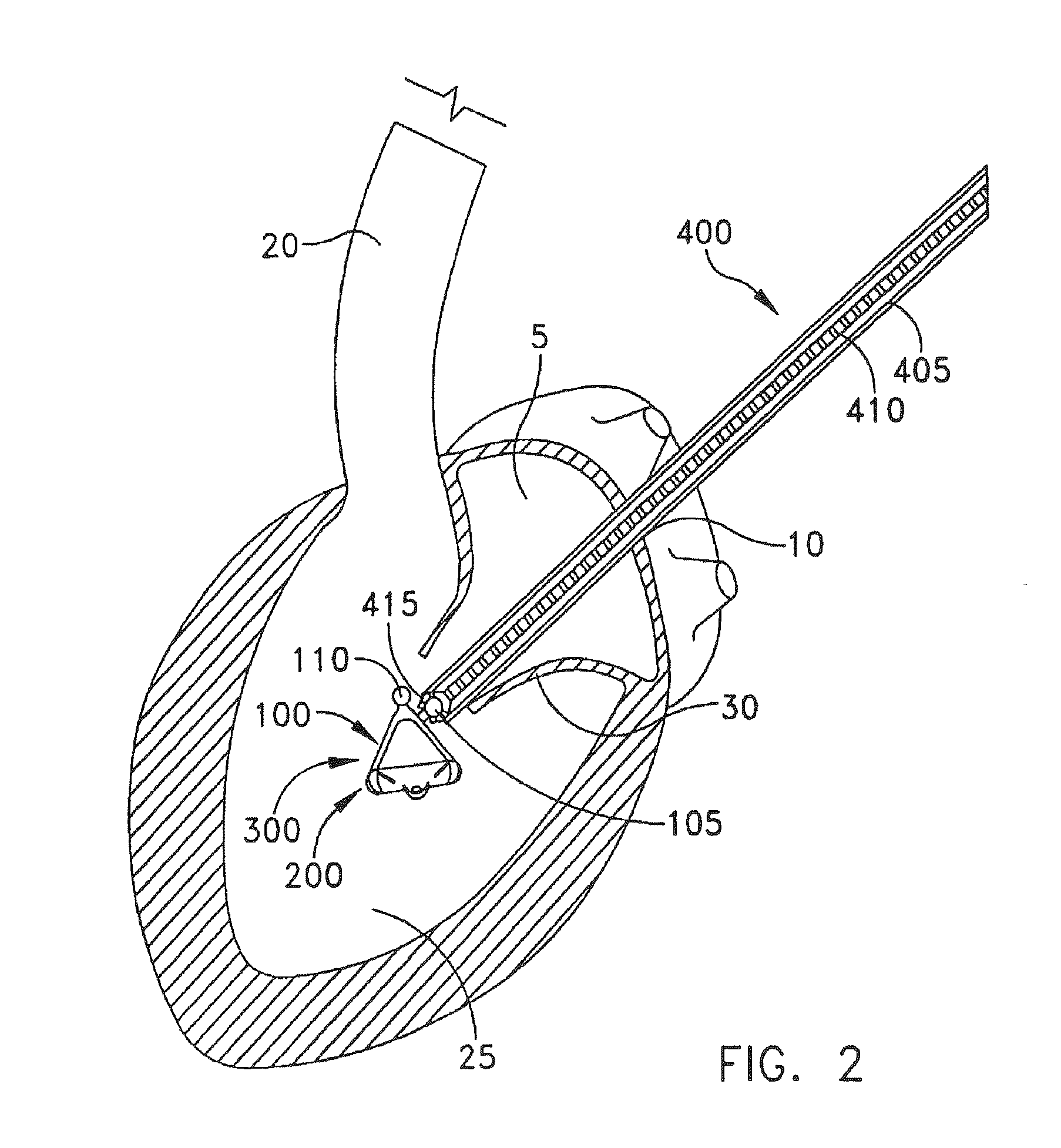
[0044]The present invention can be used to implant a variety of prostheses into the arterial system or left side of the heart. The prosthesis used in the preferred embodiment is an aortic valve prosthesis. Alternatively, the prosthesis may comprise, but is not limited to, a cylindrical arterial stent, an arterial prosthesis or graft, a ventricular assist device, a device for the treatment of heart failure such as an intraventricular counterpulsation balloon, chordae tendinae prostheses, arterial filters suitable for acute or chronic filtration of emboli from the blood stream, arterial occlusion devices and the like.
[0045]For clarity of illustration, the present invention will hereinafter be discussed in the context of implanting an aortic valve prosthesis.
[0046]It should also be appreciated that the present invention may be practiced either “on-pump” or “off-pump”. In other words, the present invention may be performed either with or without the support of cardiopulmonary bypass. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com