Process for the Preparation of Candesartan Cilexetil
a technology of candesartan and cilexetil, which is applied in the field of process for the preparation of candesartan cilexetil, can solve the problems of low yield of the process, insufficient completion of the deprotection reaction, and insufficient processing of the reaction mixture, so as to achieve short reaction time, lower impurity levels, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
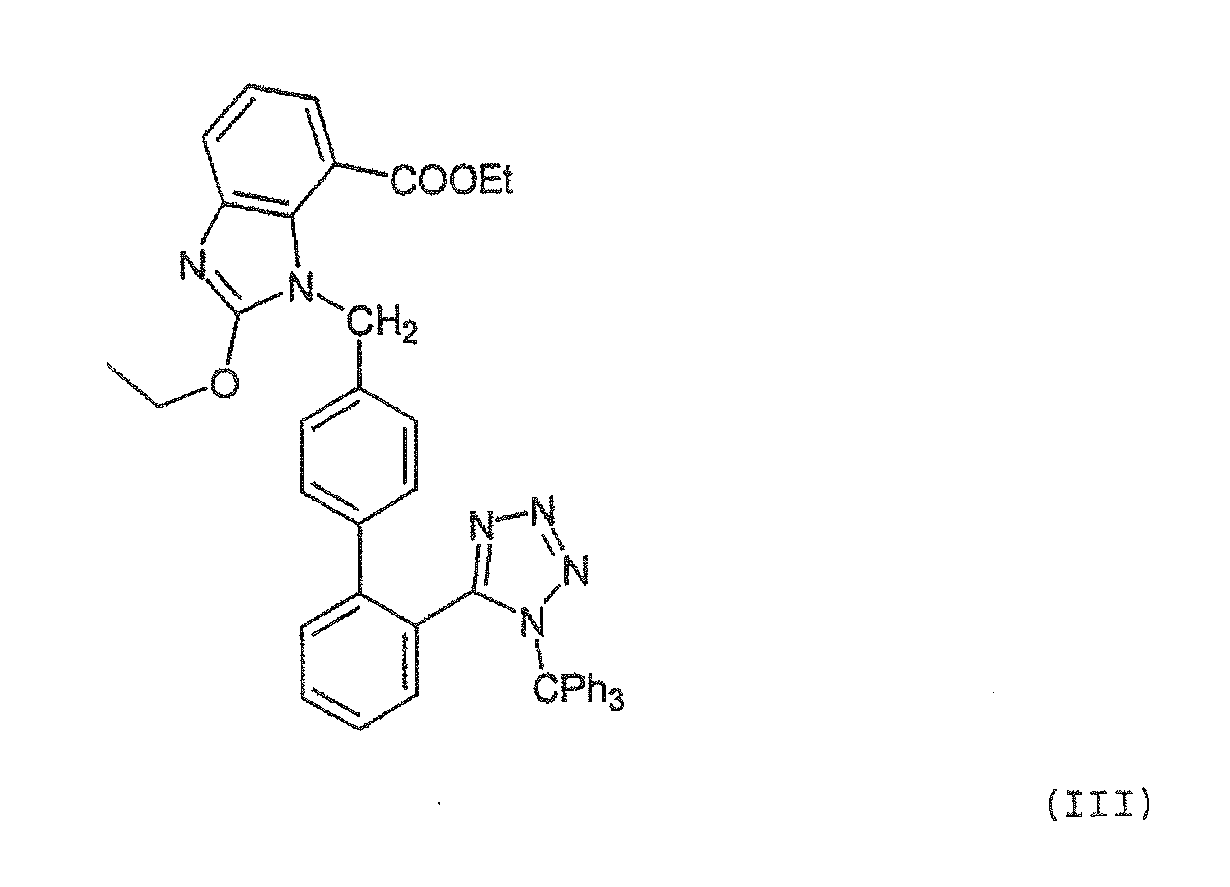
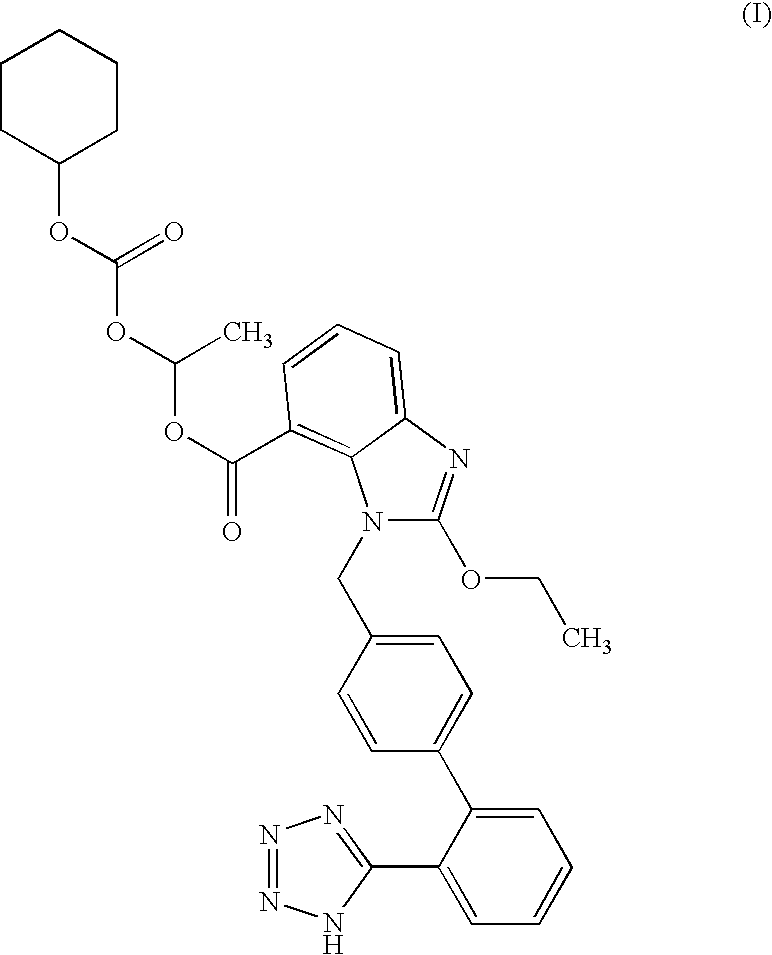
[0077]The mixture of 0.43 g (0.5 mmol) of trityl candesartan cilexetil, 15 ml of methanol, 0.05 g (0.37 mmol) of ZnCl2 and 0.4 ml of water is stirred under reflux temperature for 2.5 h. The reaction mixture is analyzed (Area % HPLC: candesartan cilexetil: 75.5%, trityl candesartan cilexetil: 1.2%, 2-oxo candesartan cilexetil: 1.6%) and cooled to room temperature. Then, the mixture is neutralized to pH 6.11 by addition of a saturated solution of NaHCO3 and methanol is evaporated. Ethyl acetate (15 ml) and water (10 ml) are added and the mixture is stirred. After the separation of phases, the organic phase is washed with 10 ml of water. The organic phase is dried over Na2SO4, filtered and evaporated to ¼ of the starting volume. To the oily remainder, 10 ml of heptane are added and cooled below 0° C. The precipitated product is collected by filtration and dried.
example 2
[0078]A mixture of 1.55 g (1.8 mmol) of trityl candesartan cilexetil, 5.4 ml of methanol, 22 ml of methylene chloride, 0.05 g (1.61 mmol) of ZnCl2 and 0.5 ml of water is stirred under reflux temperature for 5 h. The reaction mixture is analyzed (Area % HPLC: candesartan cilexetil: 76.3%, trityl candesartan cilexetil: 1.8%, 2-oxo candesartan cilexetil: 0.7%, ethyl ester of candesartan 0.09%.) and cooled to room temperature. Then, to the mixture 36 ml of methylene chloride and 55 ml of water is added. The phases were separated and organic phase is washed with 2×55 ml of water. Organic phase is dried over Na2SO4, filtered and evaporated to the oily residue. This residue is dissolved in 1.6 ml of methylene chloride and then 16 ml of isopropyl acetate is added. The mixture is stirred at 0° C. for 24 h. The precipitated product is collected by filtration and dried. After that the product was suspended in 5 ml of tert-butyl methyl ether. The mixture is stirred for 2 h. The product is colle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| w/w | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com