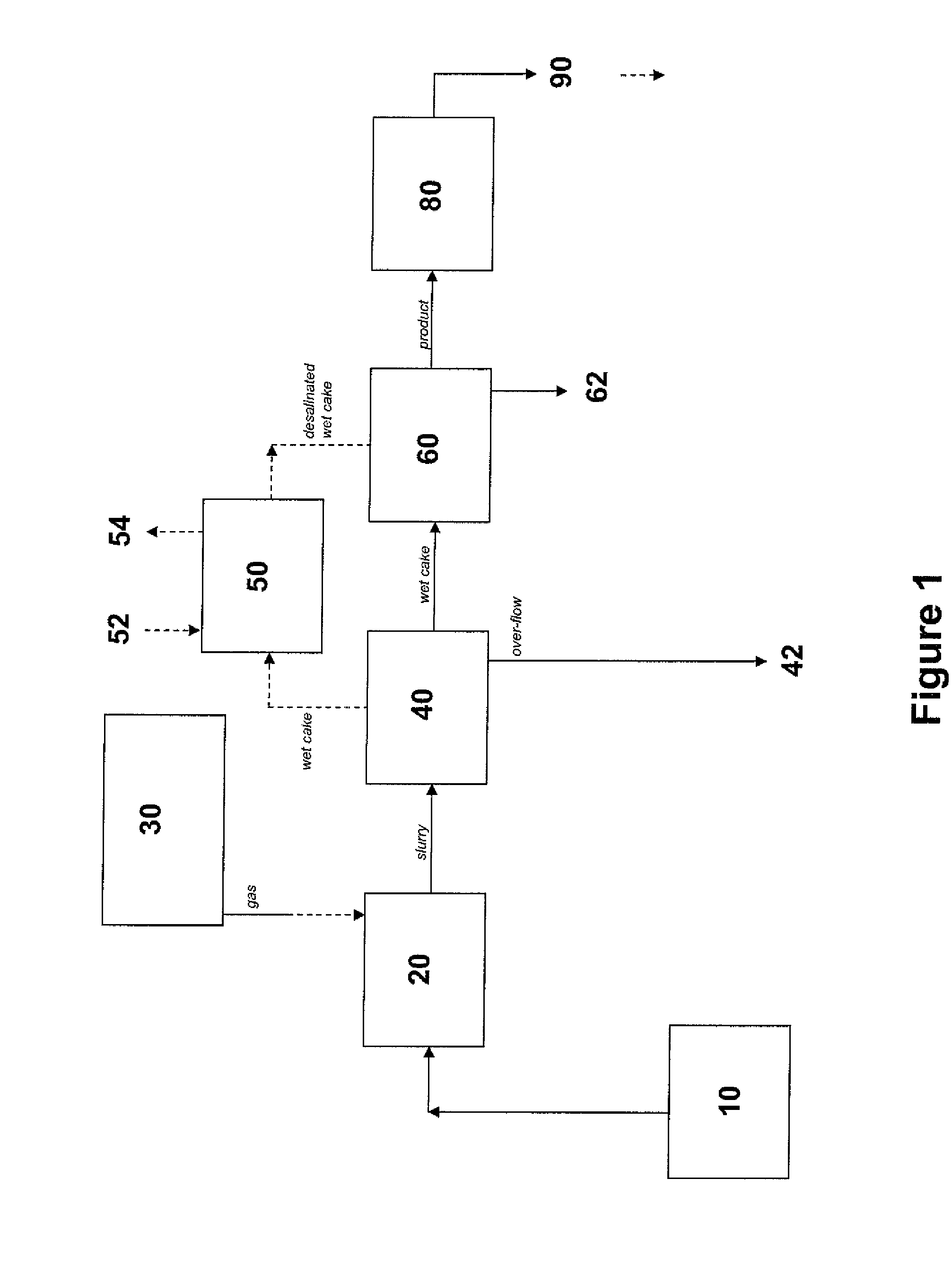
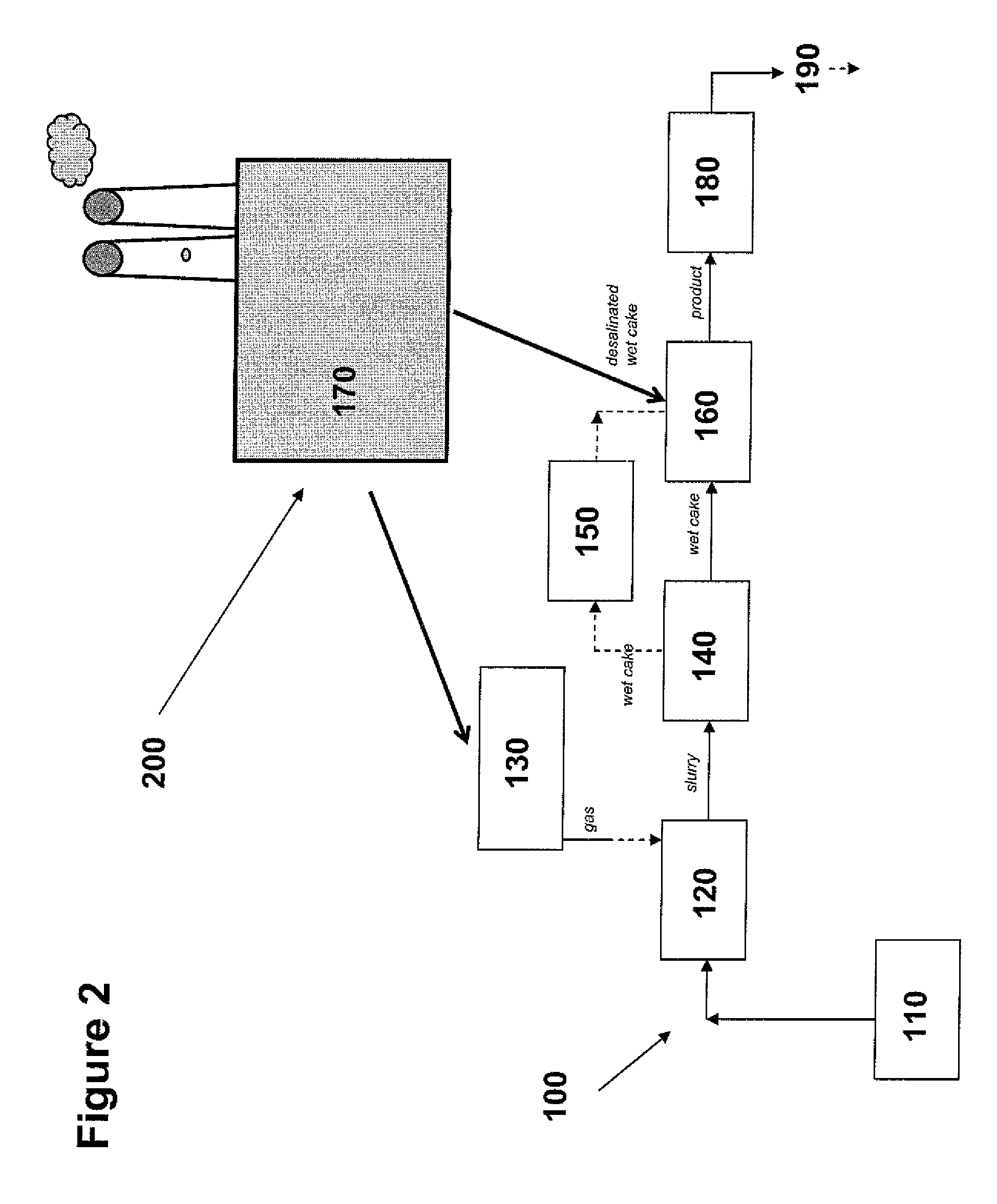
Electrochemical methods of sequestering co2
a technology of electrochemical methods and co2 is applied in the field of electrochemical methods of sequestering co2, which can solve the problems of unresolved environmental outcomes, limited capacity and duration, and economic and environmental problems, and achieve the effects of economic, environmental and social benefits, and the economic and environmental benefits of sequestering co2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Precipitation of P00099
A. P00099 Precipitation Process
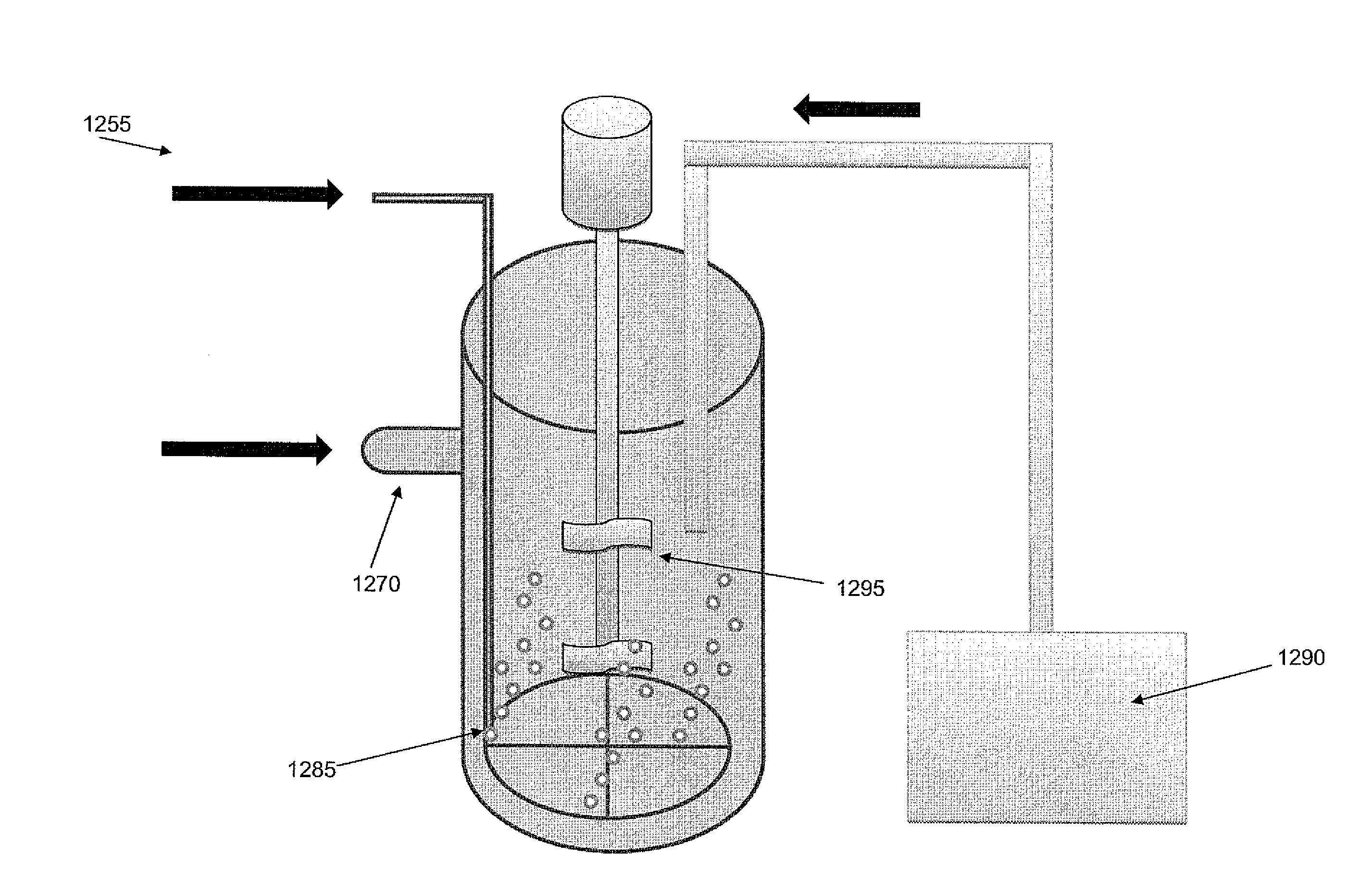
[0254]The following protocol was used to produce the P00099 precipitate. 380 L of filtered seawater was pumped into a cylindrical polyethylene 60°-cone bottom graduated tank. This reaction tank was an open system, left exposed to the ambient atmosphere. The reaction tank was constantly stirred using an overhead mixer. pH, room temperature, and water temperature were constantly monitored throughout the reaction.
[0255]25 g of granulated (Ca,Mg)O (a.k.a., dolime or calcined dolomite) was mixed into the seawater. Dolime that settled to the bottom of the tank was manually re-circulated from the bottom of the tank through the top again, in order to facilitate adequate mixing and dissolution of reactants. A second addition of 25 g of dolime was performed in an identical manner, including a manual recirculation of settled reactant. When the pH of the water reached 9.2, a gas mixture of 10% CO2 (and 90% compressed air) was slowly diffused...
example ii
Use of Fly Ash as an Alakali Source
A. Methods
[0264]500 mL of seawater (initial pH=8.01) was continuously stirred in a glass beaker using a magnetic stir bar. The pH and temperature of the reaction was continuously monitored. Class F fly ash (˜10% CaO) was incrementally added as a powder, allowing the pH to equilibrate in between additions.
B. Results and Observations:
[0265](Amounts of fly ash listed are the cumulative totals, i.e. the total amount added at that point in the experiment.)
After the additions of 5.00 g of fly ash the pH reached 9.00.
34.14 g->9.50
168.89 g->9.76
219.47 g->10.94
254.13 g->11.20
300.87 g->11.28
Much more fly ash was needed to raise the pH of the seawater than distilled water. The initial pH raise (8 to 9) required much less fly ash than the further raises. The pH remained fairly stable around 9.7 for much of the reaction. The rate of rate of pH increase went up after ˜10. Also of note was an initial drop in pH when the fly ash was added. This drop in pH is quick...
example iii
Production of High Yields
A. Process 1
[0267]A 20% CO2 / 80% Air gas mixture was sparged into 1 L of seawater until a pH 2 was added to the 1 L of carbonic acid / seawater solution. The 20 / 80 gas mixture continued to be sparged for 20 minutes to ensure maximal dissolution of the Mg(OH)2 and gases. After dissolution, sparging was stopped and 2M NaOH was added until a pH of 9.8 was reached. Sparging of the 20 / 80 gas was resumed until a pH of 8.5 was reached. 2M NaOH and counter-additions of the 20 / 80 gas were continued maintaining a pH range between 8.5 and 9.8 until a total of 200 ml of 2M NaOH was added. A yield of 6.91 g was observed having a Coulometer reading of 10.6% carbon (˜80% carbonate).
B. Process 2
[0268]A 20% CO2 / 80% Air gas mixture was sparged into 1 L of seawater until a pH 2 was added to the 1 L of carbonic acid / seawater solution. The 20 / 80 gas mixture continued to be sparged for 20 minutes to ensure maximal dissolution of the Mg(OH)2 and gases. After dissolution, sparging was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com