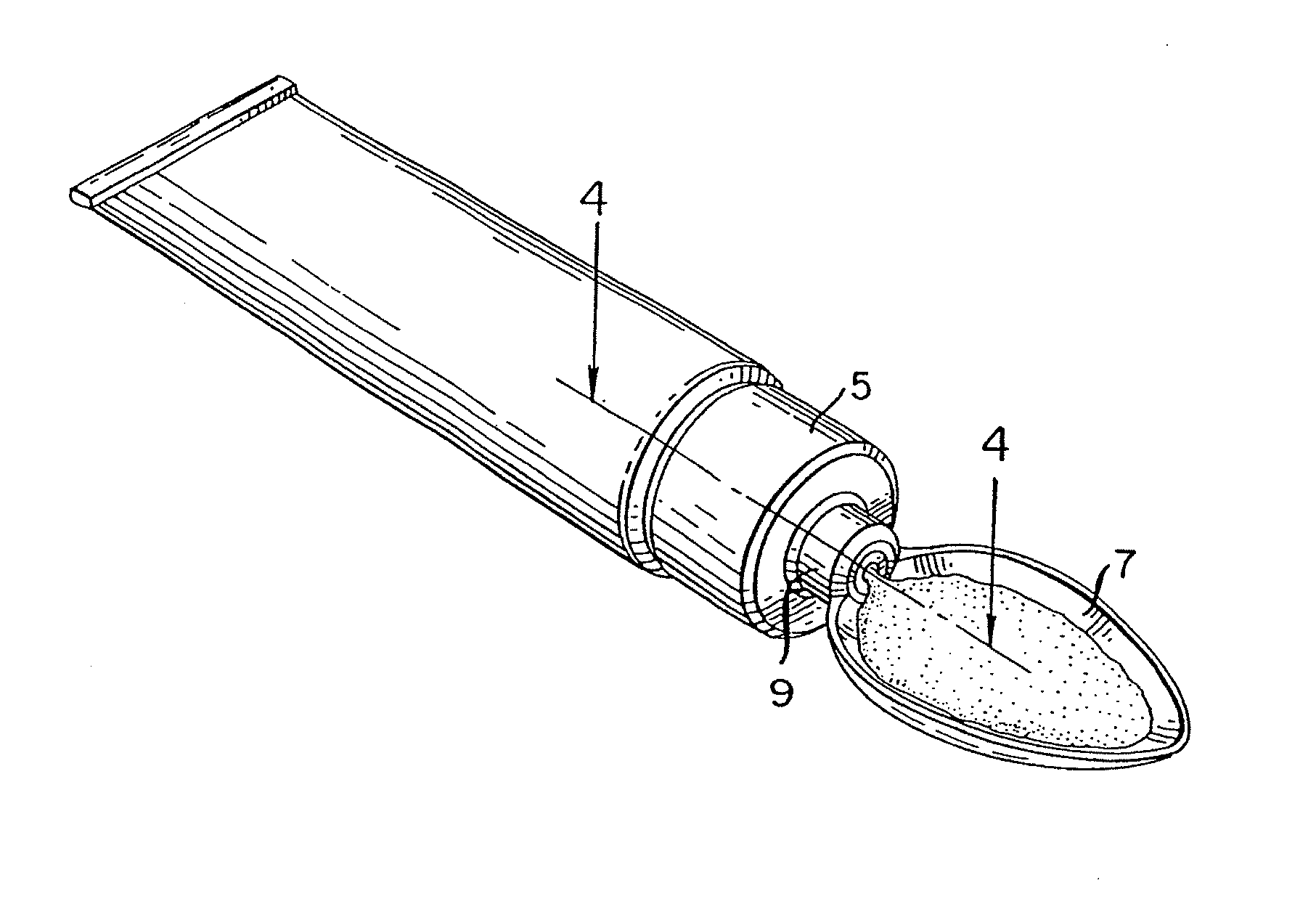
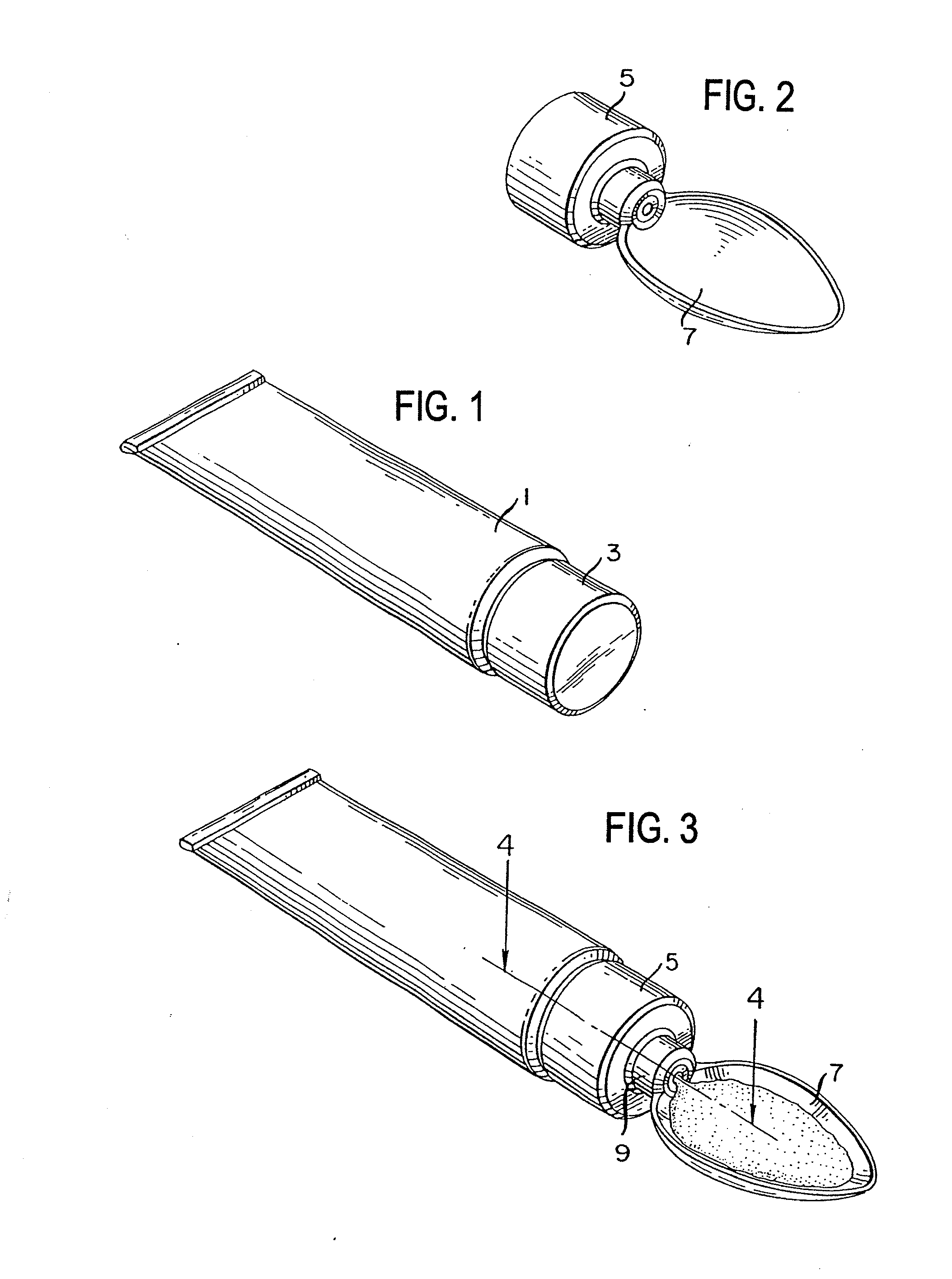
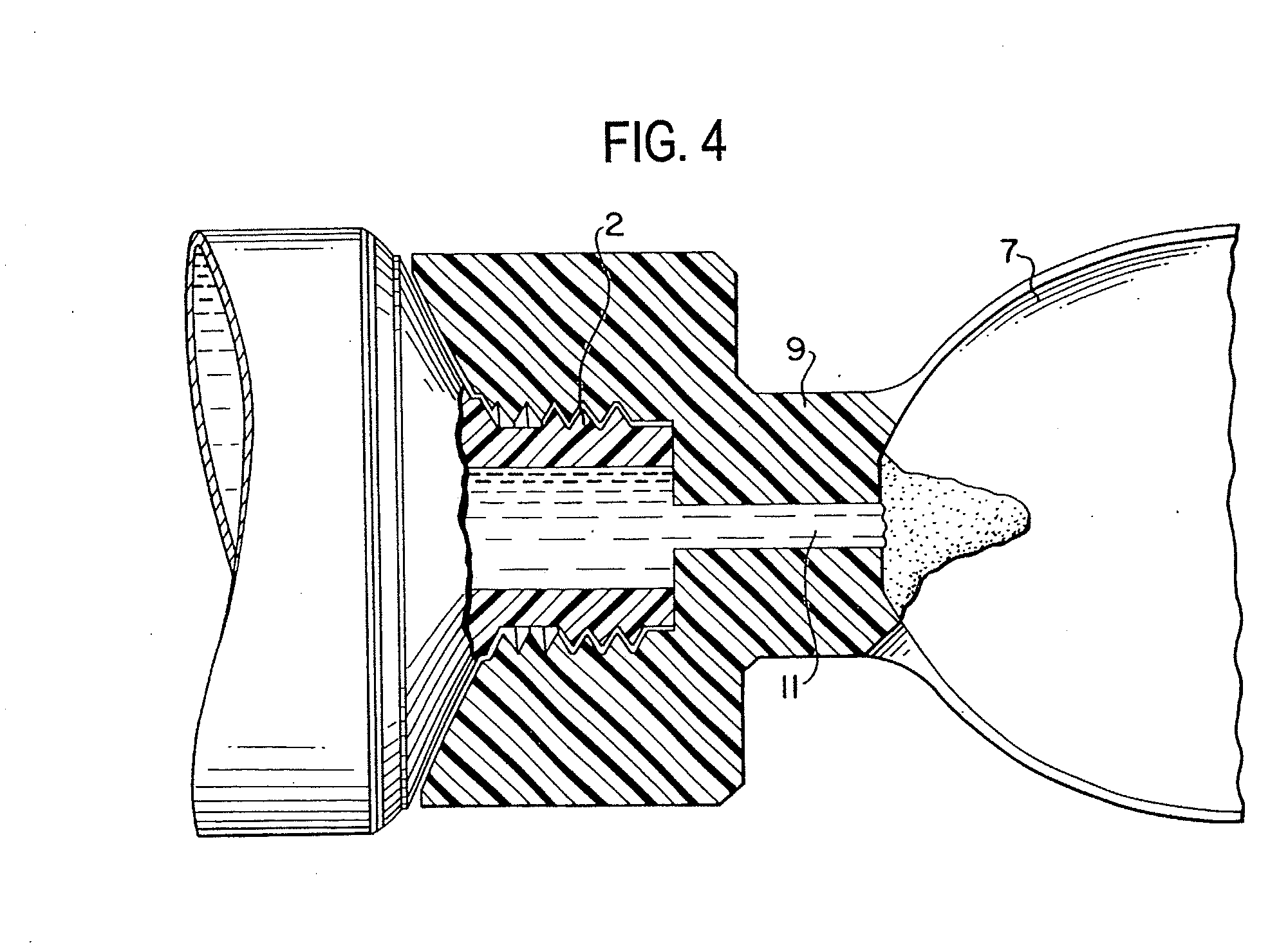
Method for administering a spill resistant pharmaceutical system
a pharmaceutical system and spill-resistant technology, applied in the field of pharmaceutical compositions for administering pharmaceuticals orally, can solve the problems of inability to accurately measure the dose, and inability to administer a prescribed dose, etc., to achieve the effect of convenient measurement, convenient swallowing, and stable components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0058]To compare the drug delivery system of the invention to other technologies, experiments were conducted with the goal of identifying and quantifying the relevant physico-chemical characteristics of formulations according to the invention. These were compared to characteristics of other formulations that are commercially available or disclosed in the prior art. The results indicate that the properties of the pharmaceutical compositions herein have surprising advantages and critical characteristics necessary for a non-spill drug delivery system and that the other tested products are unsatisfactory for this system.
[0059]Materials and Methods
[0060]TEST SAMPLES (Examples 21 to 31): Laboratory scale (100 g-500 g) batches of pharmaceutical formulations were prepared essentially according to the methods and compositions described in examples 2-4, 7, 9, 11-13, 15-16, and 18 of the earlier Ross application, U.S. Ser. No. 08 / 114,315. These compositions were tested for the new acceptance c...
example 2
Pseudoephedrine HCl Formulation Thickened with Polyethylene Glycols
[0063]
Ingredient%Pseudoephedrine HCl0.6Propylene Glycol25.0Polyethylene Glycols73.5(PEG 400:PEG 3350 3:1)Methyl Paraben0.22Sodium Saccharin0.20Strawberry Flavor0.05D&C Red #330.0057Purified water to100
[0064]PEG with a molecular weight less than 800 is a solid and works as a thickener and over 800 is a liquid. Thus, in S.N. 21, about 18% of the formulation is PEG 3350 (a liquid) and about 55.5% is PEG 400 (a thickener).
[0065]S.N. 22:
example 3
Acetaminophen Formulation Thickened with Carboxymethylcellulose
[0066]
Ingredient%Acetaminophen3.2Glycerin4.0Propylene Glycol25.0Sodium Saccharin0.2Methyl Paraben0.22Sodium Carboxymethylcellulose2.4Purified water to100
[0067]S.N. 23:
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight % | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
| start time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com