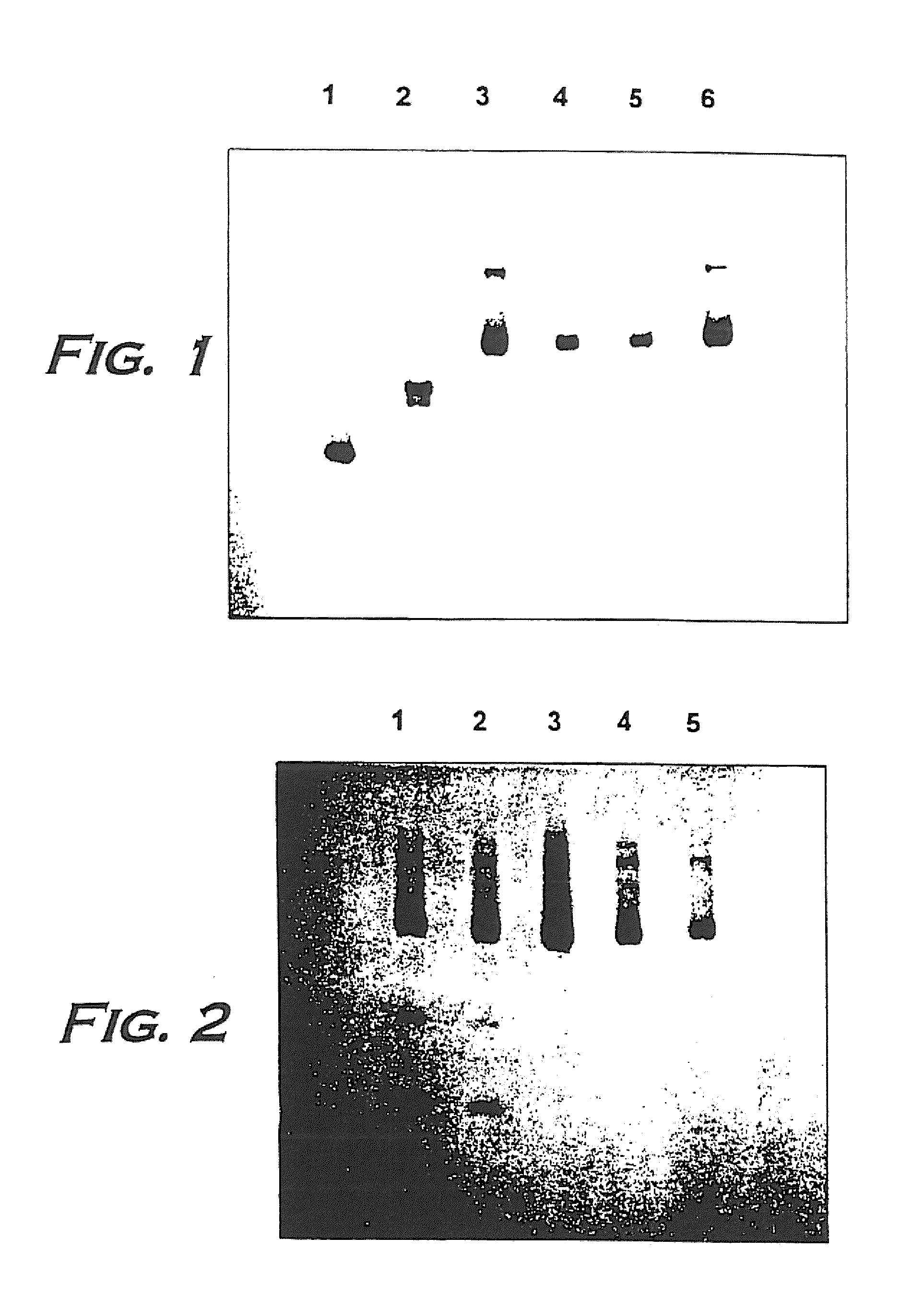
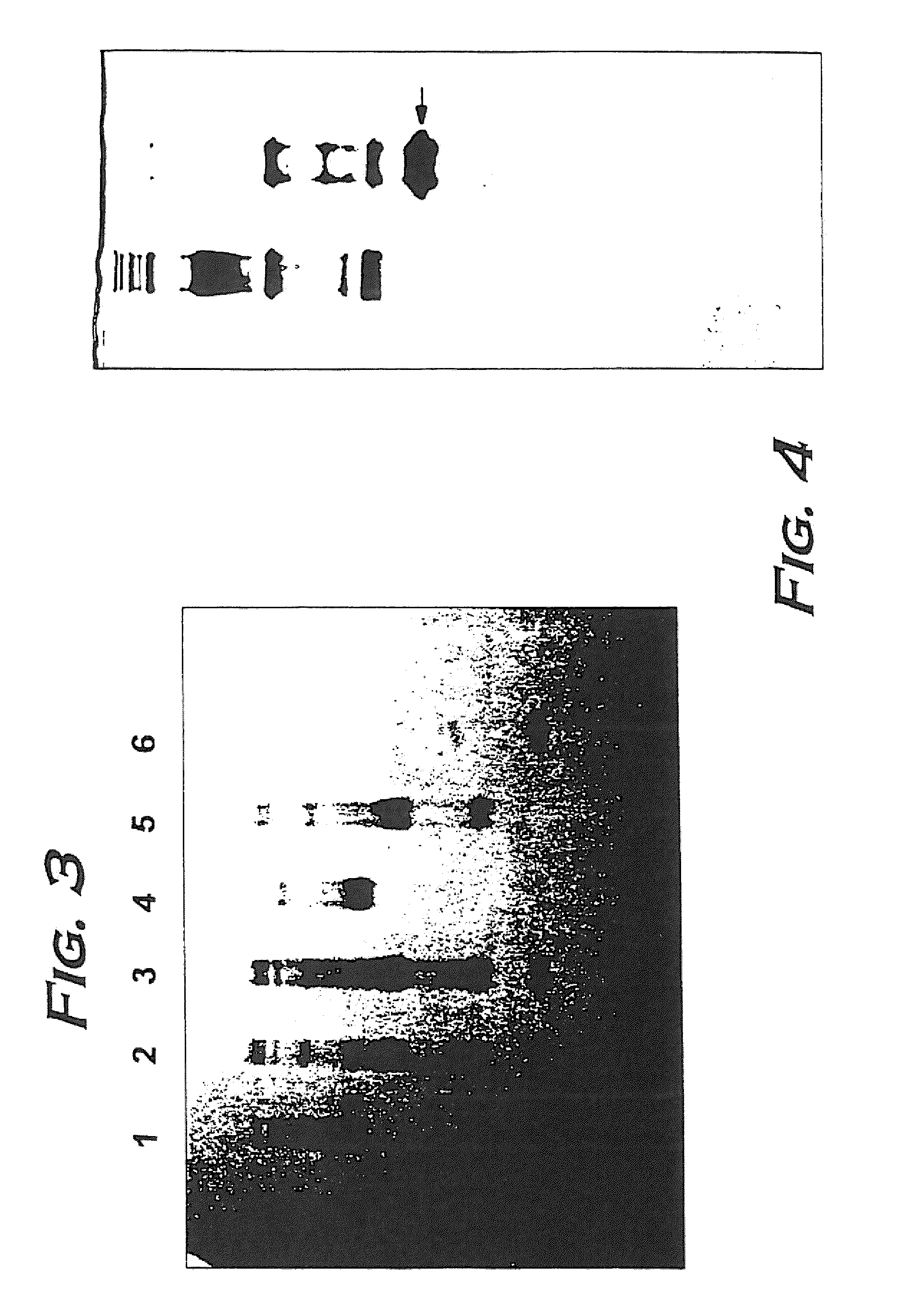
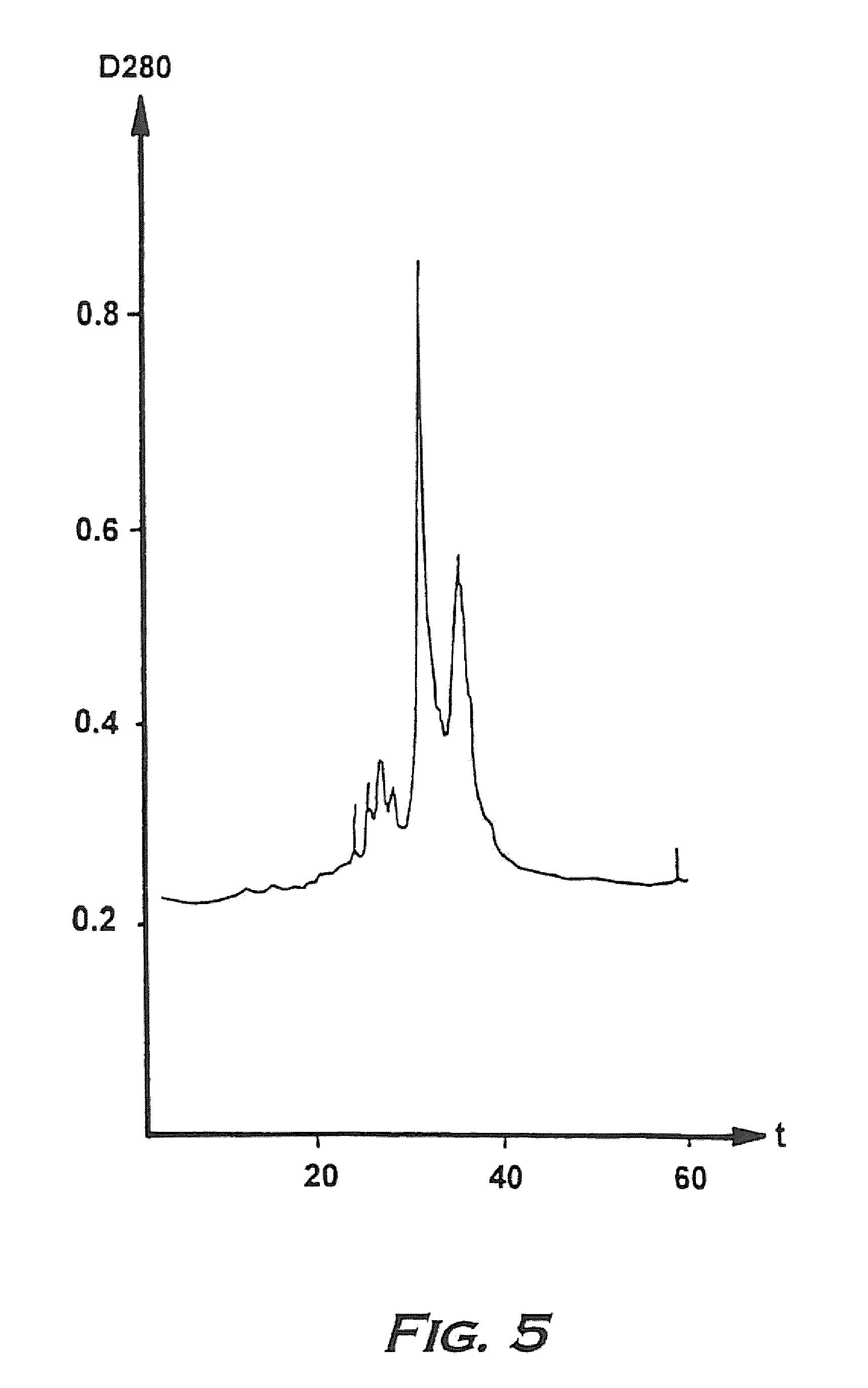
Hemoglobin alpha chain peptide fragments useful for inhibiting stem cell proliferation
a technology of hemoglobin alpha chain and peptides, which is applied in the direction of peptides, peptide/protein ingredients, peptide sources, etc., can solve the problems of no clinical use approval of this factor, bone marrow suppression or gastrointestinal toxicity,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vivo Stem Cell Proliferation Inhibition Assay
[0117]For the detection of stem cells proliferation the number of CFU-S in S-phase of the cell cycle was measured by the 3H-Thymidine “suicide” method (Becker et al., Blood 26:296-308, 1965).
[0118]Immature hematopoietic progenitors—Colony Forming Units in spleen (CFU-S)—can be detected in vivo by forming macroscopic colonies in the spleens of lethally irradiated mice, 8-12 days after the intravenous injection of hematopoietic cells (Till & McCulloch, 1961).
[0119]For the standard CFU-S proliferation assay the method of 3H-Thymidine “suicide” is usually applied (Becker et al., 1965). The method is based on incorporation of radiolabelled Thymidine, (3H-Thymidine) a precursor of DNA into cells during DNA synthesis. The CFU-S which are in S-phase of the cycle at the time of testing, are killed by the high radioactivity and therefore not able to form colonies in spleen. Thus, the difference between the number of CFU-S formed by the injection...
example 2
In Vitro Stem Cell Proliferation Inhibition Assay
[0134]Using the following test system (Lord et al., in The Inhibitors of Hematopoiesis pp. 227-239, 1987) the direct effect of INPROL was shown. The multilineage factor (IL-3) dependent stem cell line, FDCP mix A4 (A4), was maintained in IMDM medium supplemented with 20% horse serum and 10% WEHI-3-conditioned medium as a source of colony-stimulating IL-3.
[0135]A tritiated Thymidine incorporation assay was used to measure proliferation: A4 cells (5×104 in 100 μl medium with 20% horse serum and 50% of WEHI-3 CM) were incubated at 37° C. in 5% CO2 for 16 hours.
[0136]pINPROL or the crude BME (fraction IV) were added at the start. Tritiated thymidine ((3H-Tdr) 3.7 KBq in 50 μl at 740 GBq / mmole) was then added to each group for a further 3 hours of incubation. The level of proliferation was measured by harvesting cells and the % inhibition calculated using the formula
%Inhibition=cpmwithoutINPROL-cpmwithINPROLcpmwithoutINPROL×(100%)
[0137]Inc...
example 3
Inhibition of CFU-S Proliferation by INPROL Injected In Vivo
Doses and the Duration of the Effect
[0138]The studies of the effect of INPROL injected in vivo revealed that INPROL can effectively block the recruitment of CFU-S into cycle, thus protecting those cells from the cytotoxic effect of further treatment, showing its potential for clinical use.
[0139]The experimental protocol had two goals: to check the effect of INPROL on CFU-S when injected in vivo and to define the effective duration of INPROL activity in relation to cycling stem cells.
[0140]To stimulate CFU-S proliferation, the injection of testosterone-propionate was used based on the effect mentioned above in Example 1.
[0141]Mice BDF1 were injected with TSP (10 mg / 100 g) on Day 0; 24 hours later mice of each experimental group (4 mice per group) received a single pINPROL injection at doses of 0 μg, 5 μg, 10 μg, and 15 μg / mouse i.p.
[0142]Twenty-four hours after pINPROL injection, mice were sacrificed and the percent of cycli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com