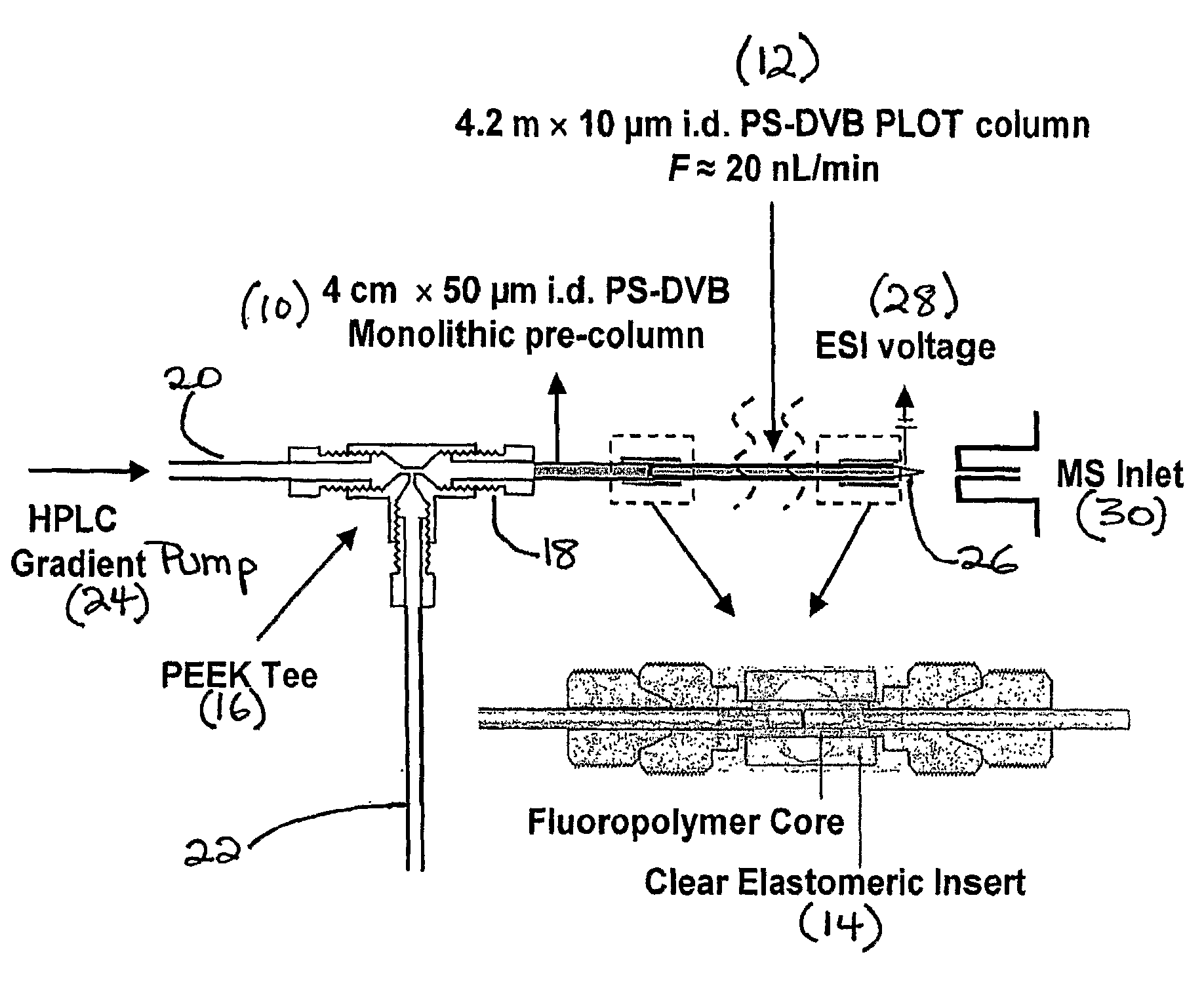
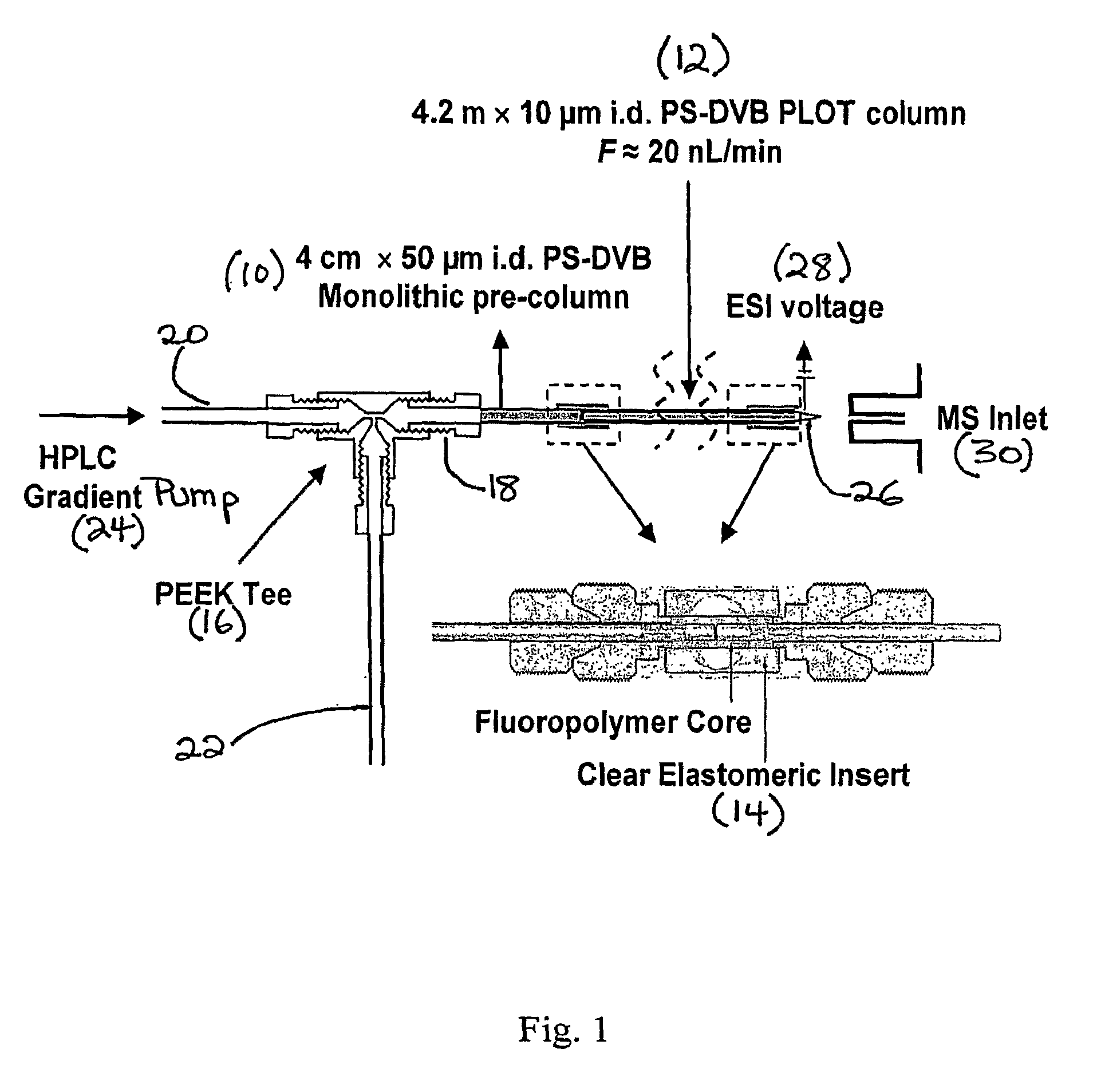
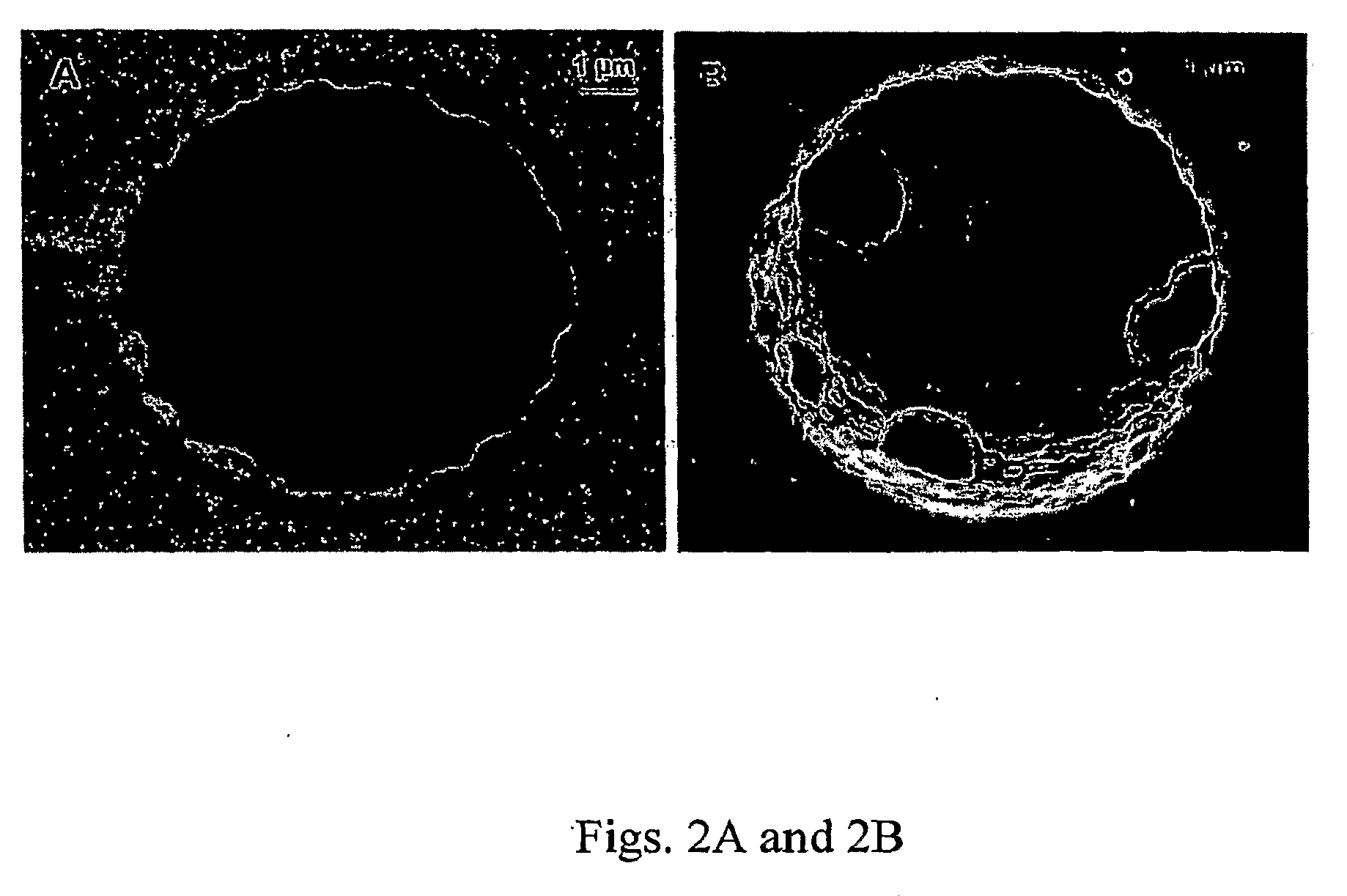
Narrow bore layer open tube capillary column and uses thereof
a capillary column and narrow bore technology, applied in the direction of isotope separation, diaphragm, electrolysis, etc., can solve the problems of difficult preparation of monolithic columns, low retention and sample loading capacity, and difficult lc-ms analysis of very small quantity samples, etc., to achieve high column-to-column reproducibility, high resolving power, and high resolving power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Preparation and Characterization of a PLOT Column According to the Invention
[0032]Fused-silica capillary tubing with a 10 μm i.d. (˜5 meters) was first flushed overnight with 1.0 mol / L NaOH at ˜1000 psi, washed with water and flushed with 1.0 mol / L hydrochloric acid, and then washed again with water and acetonitrile. The capillary was dried with nitrogen at ˜1000 psi to remove residue water and acetonitrile. 30% (v / v) 3-(trimethoxysilyl)propyl methacrylate and 0.5% (wt / v) 2,2′-diphenyl-1-picrylhydrazyl (DPPH) in N,N-dimethylformamide anhydrous (DMF) was freshly prepared and filled into the 10 μm i.d. pretreated capillary. Both ends of the capillary were sealed with a septum, and the capillary was placed in an oven at 110° C. for 6-10 h. The capillary was washed with acetonitrile and blown dry with nitrogen at 1000 psi. A polymerization solution was prepared containing of 5 mg of AIBN, 200 μL styrene, 200 μL DVB, and 600 μL ethanol. The solution was degassed by ultrasonication for 5 ...
example ii
Characterization of Column Performance
[0040]A variety of chromatographic studies were conducted to characterize a 4.2 m×10 μm i.d. PLOT column according to the invention. The long column provided a flow rate of ˜20 nL / min at pressures of only ˜2900 psi. on the basis of these conditions, Darcy's law41 was used to calculate the column permeability as 1.3×10−2 m2. It is interesting to note that this value is roughly 4-fold lower than an equivalent open tube capillary of 10 μm i.d. without a porous layer. The lower permeability and higher pressure drop of the PLOT column according to the invention is undoubtedly due to the porous layer reducing the open tube diameter. On the other hand, the permeability is 15-fold higher than a recently introduced 10 μm i.d. silica monolithic column16. Thus, the column according to the invention is characterized by relatively high permeability in comparison to a similarly sized packed column. Hence, very long columns according to the invention, e.g., of...
example iii
Comprehensive Analysis of Large Complex Peptide Fragments of EGFR
[0047]High sequence coverage proteomic analysis and comprehensive characterization of post-translational modifications at the trace level are particularly important to help to address a variety of problems of biological interest. Often, one is faced with a limited amount of sample and, yet it can be important to determine and quantitate individual protein isoforms. An intermediate approach between top down and bottom up proteomics, extended range proteomic analysis (ERPA), was recently introduced for comprehensive characterization of complex proteins44. Lys-C was used as the proteolytic enzyme instead of trypsin, since the former enzyme is a less frequent cutter. Thus, the complexity of the sample was reduced (˜2-3 fold lower number of peptide fragments than for trypsin). The Lys-C digest, on average, led to longer peptides than that for the tryptic digest. In addition, extra arginines were frequently included in the d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com