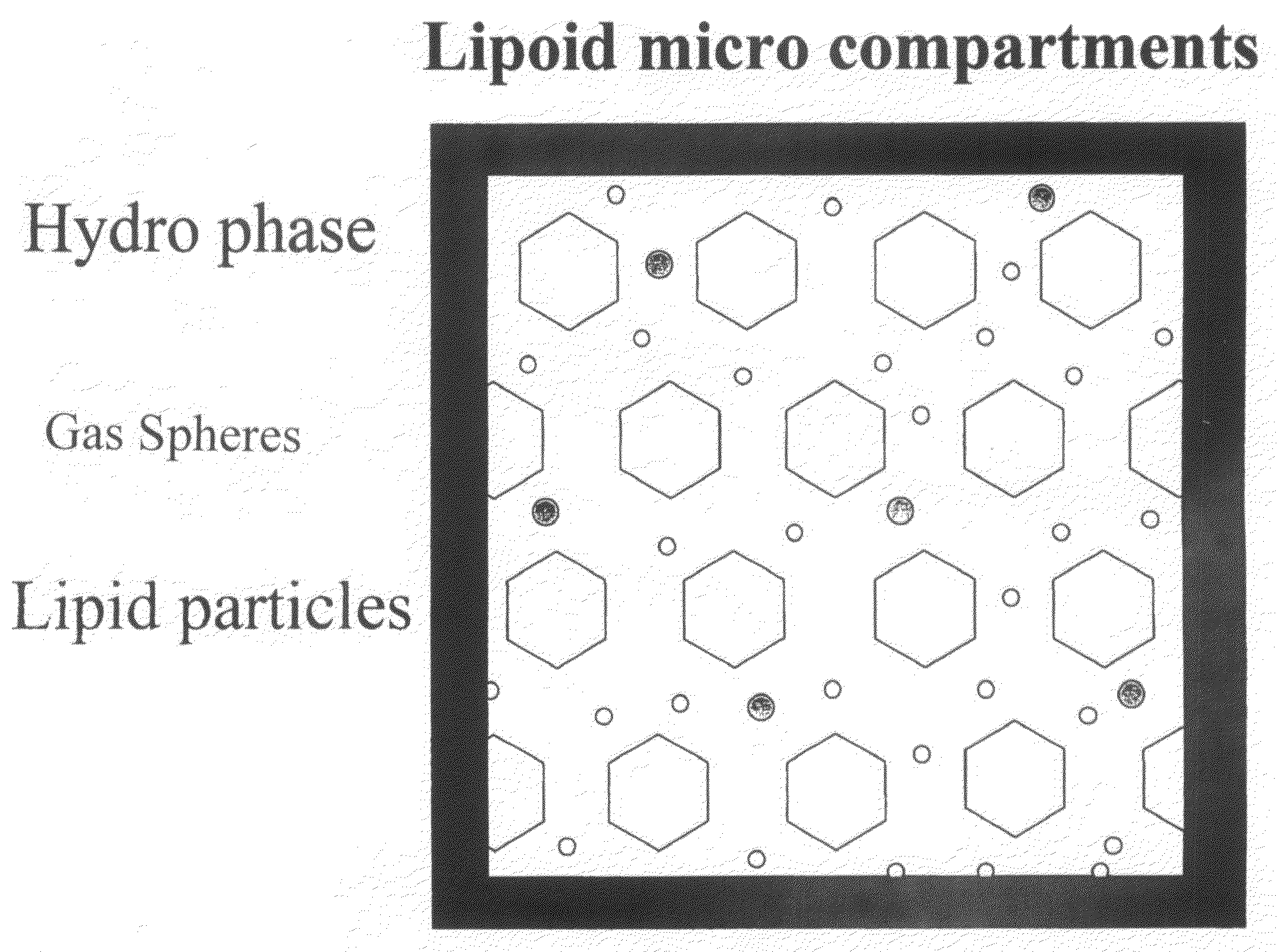
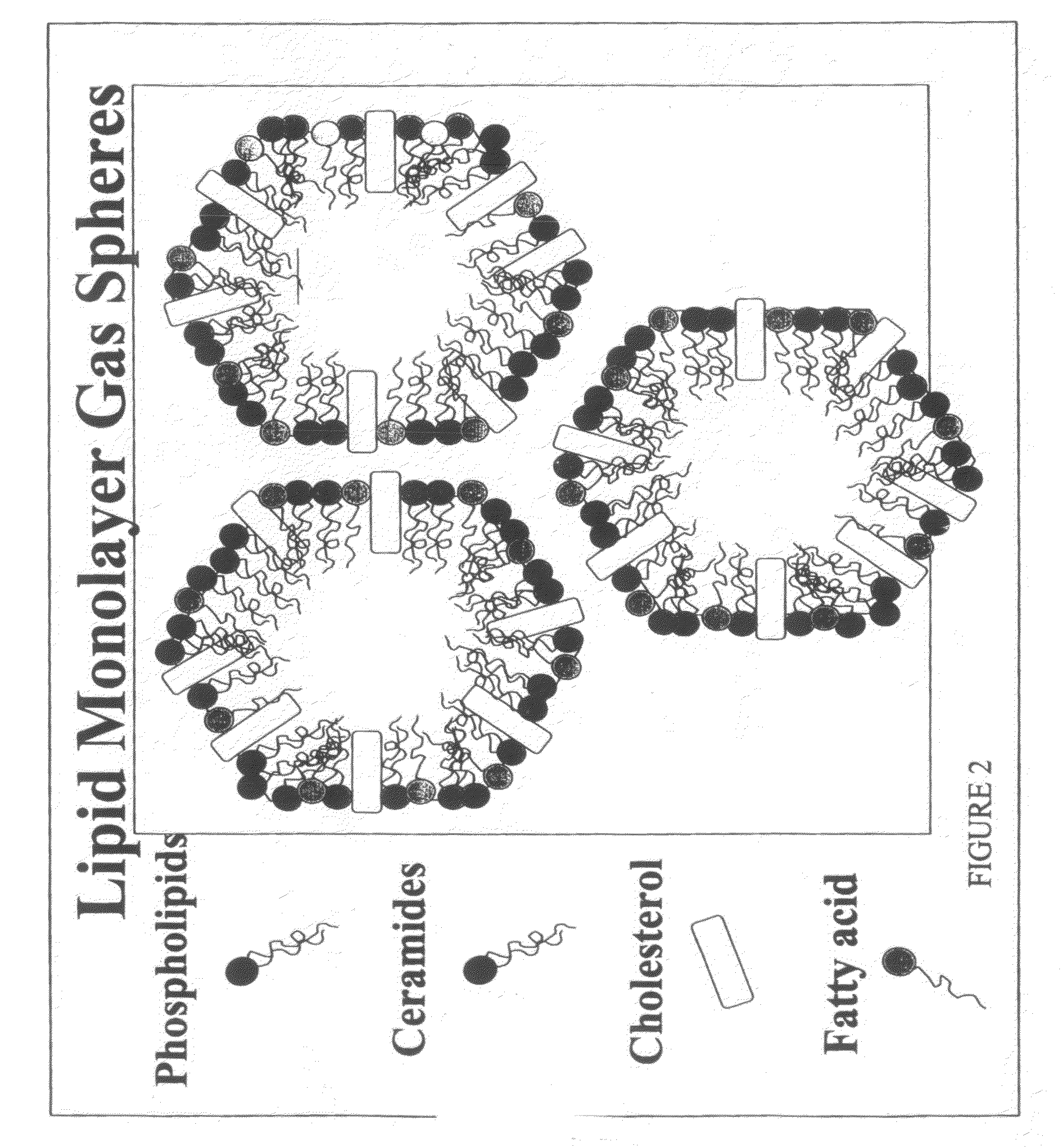
Water-based delivery systems
a delivery system and water-based technology, applied in the field of new products, can solve the problems of stratum corneum losing its natural ability to function properly as a barrier, skin becoming either too dry or too permeable to environmental substances, and achieve the effect of enhancing skin barrier restoration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
A General Method of Making
[0154]The phospholipid, cholesterol, palmitic acid and ceramide components are mixed together with water, and agitated at a temperature of 70-80° C. The following additional components are added: mevalonic acid lactone, 25-hydroxycholecalciferol, propylene glycol, glycerine, PVP, TEA added along with water, and sodium hydroxide. Sodium hydroxide is added to adjust viscosity and stabilize the formulation. Water is then added, and the formulation is agitated well. The formulation is then cooled down.
[0155]An active substance can be dissolved in both the lipid phase and / or the water phase, depending on the solubility and concentration of the active substance.
example 2
Formulation of a Preferred Embodiment of the Topical Delivery System
[0156](An active ingredient is excluded from this formulation.)
ComponentTotal amountWater79.5% of formulation Epikuron 200SH3.5% of formulationPalmitic acid1.5% of formulationCholesterol1.5% of formulationMevalonic acid0.01% of formulation Triethanolamine0.5% of formulationPhenonip0.4% of formulationXanthan gum2.0% of formulationSkinflux2.0% of formulation25-hydroxycholecalciferol0.0015% of formulation Propylene glycol4.0% of formulationGlycerol3.0% of formulationPolyvinylpyrrolidone2.0% of formulationEpikuron 200SH are hydrogenated lecithins, i.e. phosphatidylcholine (PC).“Skinflux” is a blend product obtainable from Degussa Goldschmidt which contains: Ceramide 1, 3, 6II; Phytosphingosine; Cholesterol; Sodium Lauroyl Lactylate; Carbomer; and Xanthan Gum.Mevalonic acid lactone is a lipid precursor for cholesterol / fatty acids.25-Hydroxycholecalciferol is a lipid precursor for ceramidesXanthan Gum is a thickener (pol...
example 3
Formulation with Lidocaine as an Active Ingredient
[0166]An example of a 48 kg batch of a formulation of the delivery system follows. The three fractions used to prepare this formulation each contain 16 kg.
INCI NameTrade NameSupplierCASAmountHydrogenatedEpikuron 200 SHDegussa1.7 kgLecithinesGoldschmidtCholesterolVendico57-88-50.8 kgPalmitic acidKarlshamn57-10-30.8 kgCeramide 1, 3, 6II,Skin FluxDegussa1.0 kgPhytosphingosine,GoldschmidtCholesterol,Sodium LauroylLactylate,Carbomer, XanthanGum.Mevalonic acidSigma Aldrich674-26-04.8 g lactone25-Hydroxy-Solvay19356-17-30.72 kg cholecalciferolPropylene glycolMB-Sveda57-55-62.0 kgGlycerin, 99.5%Vendico56-81-51.5 kgPolyvinylpyrrolidoneApoteket9003-39-81.0 kgXanthan gumSigma Aldrich11138-66-21.0 kgTriethanolamine,MB-Sveda102-71-60.3 kg85%PhenonipVendico0.2 kgChemicalLidocainUSP-gradeApoteket2.4 kgPurifiedUp toWater 48 kg
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com