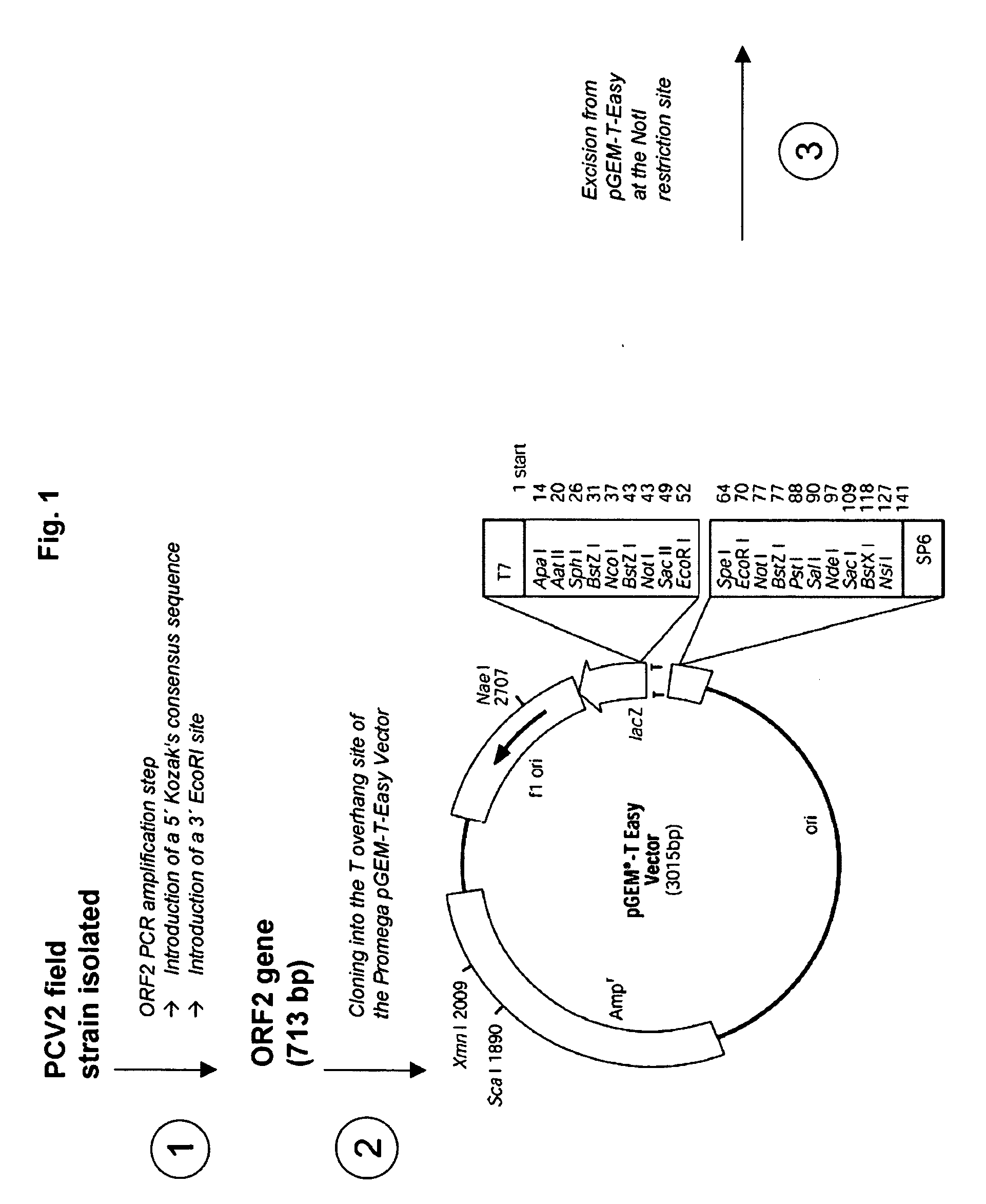
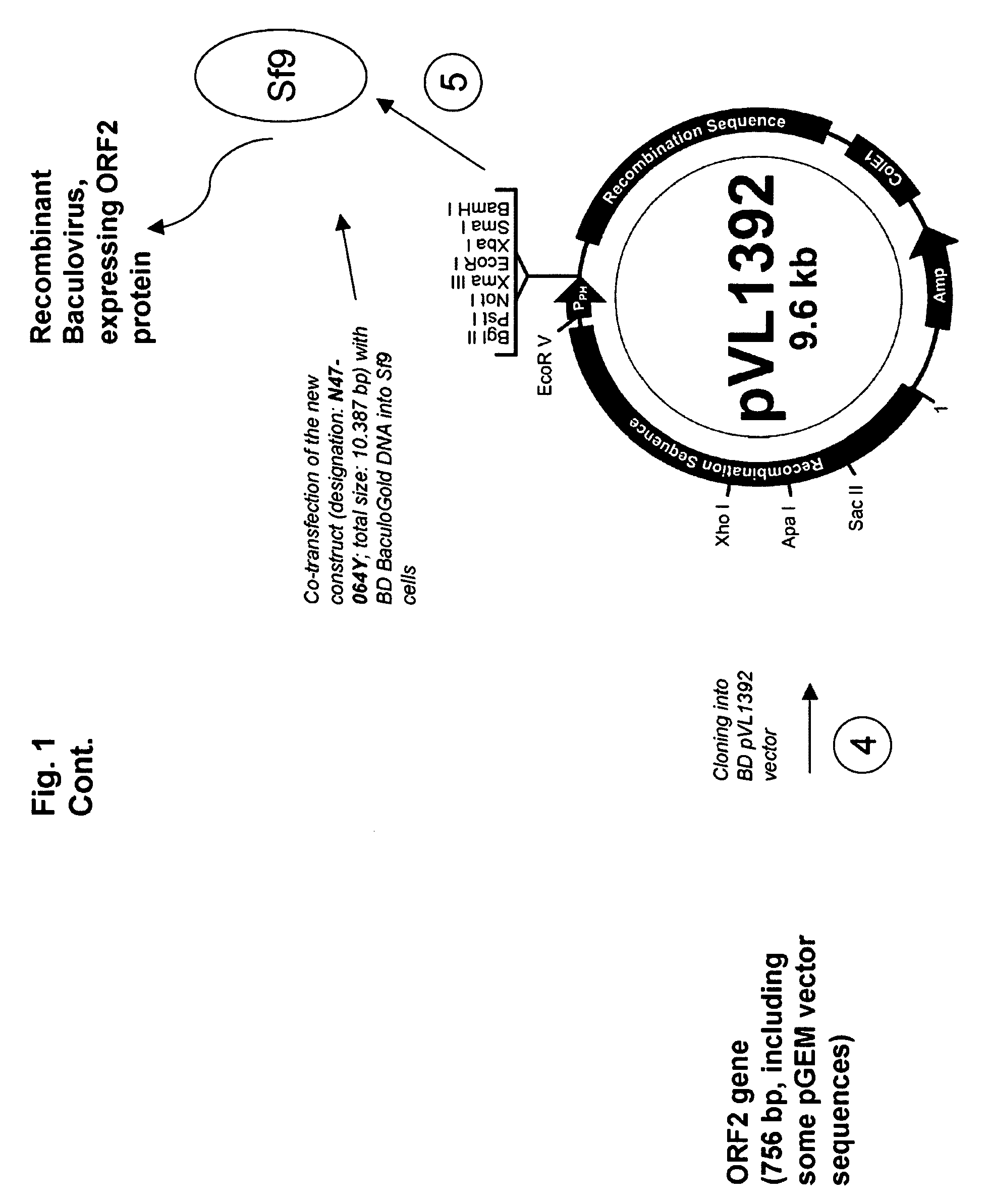
Pcv2 immunogenic compositions and methods of producing such compositions
a technology of immunogenic compositions and compositions, applied in the direction of immunological disorders, antibody medical ingredients, peptide sources, etc., can solve the problems of ineffective vaccines, high extraction procedures, and low amount of orf2 recovered from cells, and achieve the effect of lessening this
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0093]This example compares the relative yields of ORF2 using methods of the present invention with methods that are known in the prior art. Four 1000 mL spinner flasks were each seeded with approximately 1.0×106 Sf+ cells / ml in 300 mL of insect serum free media, Excell 420 (JRH Biosciences, Inc., Lenexa, Kans.). The master cell culture is identified as SF+(Spodoptera frugiperda) Master Cell Stock, passage 19, Lot#N112-095W. The cells used to generate the SF+ Master Cell Stock were obtained from Protein Sciences Corporation, Inc., Meriden, Conn. The SF+ cell line for this example was confined between passages 19 and 59. Other passages will work for purposes of the present invention, but in order to scale the process up for large scale production, at least 19 passages will probably be necessary and passages beyond 59 may have an effect on expression, although this was not investigated. In more detail, the initial SF+ cell cultures from liquid nitrogen storage were grown in Excell 420...
example 2
[0098]This example provides data as to the efficacy of the invention claimed herein. A 1000 mL spinner flask was seeded with approximately 1.0×106 Sf+ cells / ml in 300 mL of Excell 420 media. The flask was then incubated at 27° C. and agitated at 100 rpm. Subsequently, the flask was seeded with 10 mL of PCV2 ORF2 / Bac p+6 (the recombinant baculovirus containing the PCV2 ORF2 gene passaged 6 additional times in the Sf9 insect cells) virus seed with a 0.1 MOI after 24 hours of incubation.
[0099]The flask was then incubated at 27° C. for a total of 6 days. After incubation, the flask was then centrifuged and three samples of the resulting supernatant were harvested and inactivated. The supernatant was inactivated by bringing its temperature to 37±2° C. To the first sample, a 0.4M solution of 2-bromoethyleneamine hydrobromide which had been cyclized to 0.2M binary ethlylenimine (BEI) in 0.3N NaOH is added to the supernatant to give a final concentration of BEI of 5 mM. To the second sample...
example 3
[0101]This example demonstrates that the present invention is scalable from small scale production of recombinant PCV2 ORF2 to large scale production of recombinant PCV2 ORF2. 5.0×105 cells / ml of SF+ cells / ml in 7000 mL of ExCell 420 media was planted in a 20000 mL Applikon Bioreactor. The media and cells were then incubated at 27° C. and agitated at 100 RPM for the next 68 hours. At the 68th hour, 41.3 mL of PCV2 ORF2 Baculovirus MSV+3 was added to 700 mL of ExCell 420 medium. The resultant mixture was then added to the bioreactor. For the next seven days, the mixture was incubated at 27° C. and agitated at 100 RPM. Samples from the bioreactor were extracted every 24 hours beginning at day 4, post-infection, and each sample was centrifuged. The supernatant of the samples were preserved and the amount of ORF2 was then quantified using SDS-PAGE densitometry. The results of this can be seen in Table 3 below:
TABLE 3Day after infection:ORF2 in supernatant (μg / mL)429.33541.33631.33760.67...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com