Method of producing tissue by placing a molding support within a body cavity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Creation of an Artificial Blood Vessel
[0113]The first step in creating an artificial blood vessel was to determine an appropriate implant material which would:
[0114](i) initiate granulation tissue development;
[0115](ii) be covered by mesothelium;
[0116](iii) form a tube-like structure;
[0117](iv) be of variable diameter and length;
[0118](v) not attach to omentum / mesentery in the peritoneal cavity; and
[0119](vi) allow its own easy removal from the granulation tissue.
[0120]The second step was to determine its optimal time for harvest.
[0121]a) Selection of Appropriate Material as an Arterial Template
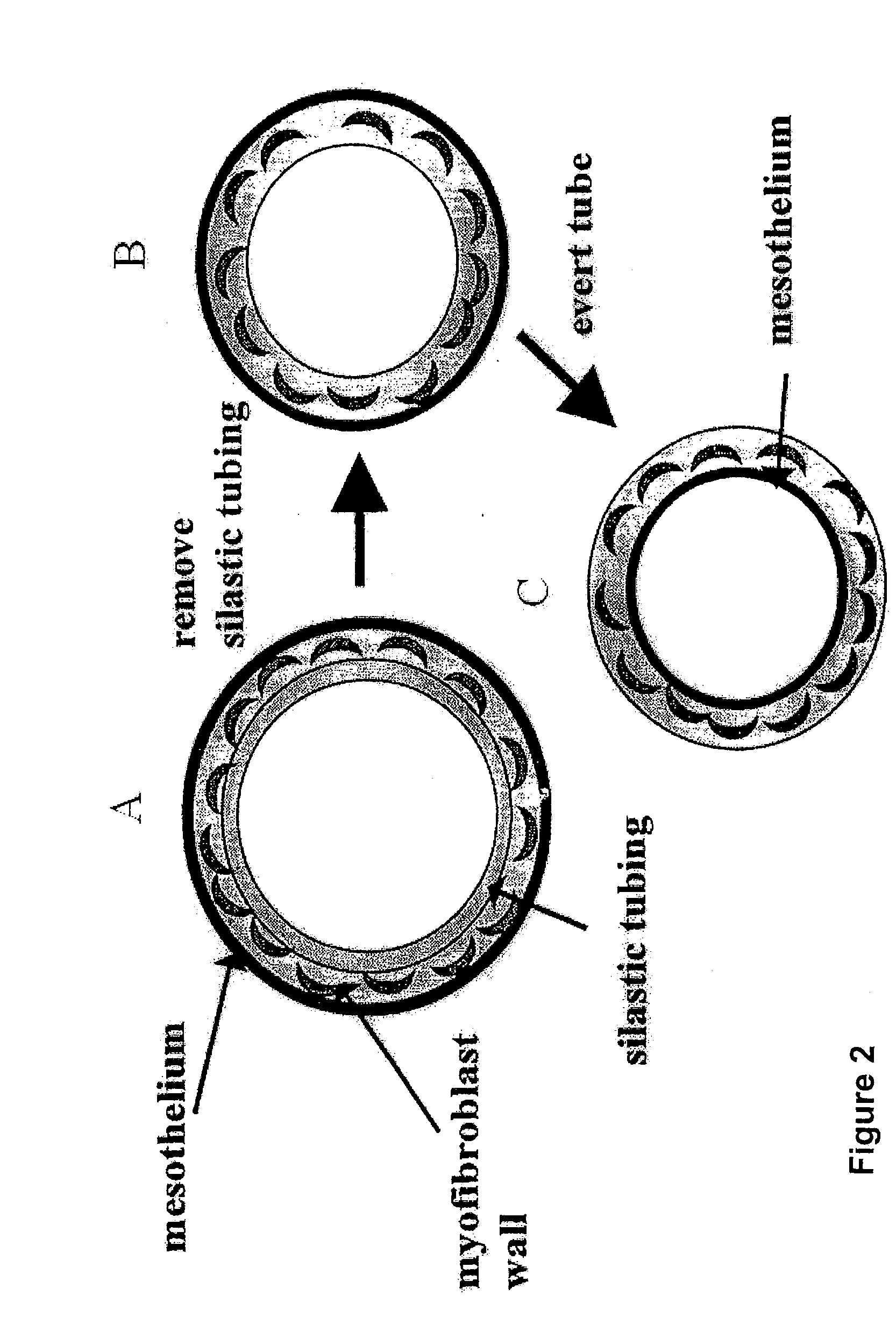
[0122]Twenty male adult Wistar rats were anaesthetized with 2.5% v / v (O2) halothane. A 20 mm incision was made in the shaved abdominal wall and a variety of objects—plastic silastic tubing (inner diameter range from 0.5-5 mm), glass rod, expanded polytetrafluoroethylene (ePTFE) graft (inner diameter 5 mm) and polyethylene terephthalate (DACRON) graft (inner diameter 6 mm)—inserted inside the ...
example 2
Myofibroblast Tubes can be Grown to Different Lengths and in Different Species
[0132]Having established that silastic tubing is a suitable mould to produce a myofibroblast tube, that tubes of different diameter (0.5 to 5 mm) could be produced, and that 2 weeks is the optimal period for their development within the peritoneal cavity, the inventors next determined whether artificial arteries could be produced in a species other than the rat, and whether these vessels could be longer.
[0133]Four pieces of silastic tubing with outer diameter of 3 mm and length 10 mm (rat) and 5 mm by 20 mm (rabbit) were placed inside each animal. Five male Wistar rats and five male New Zealand White Cross rabbits were used. Two weeks after graft placement, animals were sacrificed. The silastic tubing was carefully removed and the tube of tissue gently everted such that the mesothelial layer now lined the inside of the freed myofibroblast tube. Segments of the four myofibroblast tubes (free-floating) from ...
example 3
Macrophages are the Source of Myofibroblasts / Smooth Muscle in the Peritoneal Tubes of Tissue
[0139]a) In Vitro Studies
[0140]Cultures of macrophage cell lines (RAW 264 and J774) were grown in Dulbecco's Modified Essential Medium (Gibco) and 10% v / v foetal calf serum at 371 C in 6% v / v CO2 humidified incubators. At sub-confluency, 25 U / ml of γ-interferon (Holan Biotechnology) was added to the macrophages and incubated for 16 hours. This cytokine caused de novo expression of SM α-actin proteins in both the RAW 264 (16%) and J774 (13.5%) macrophages, as measured by Western blotting (Hartig et al, 1997 supra) [FIG. 7].
[0141]This experiment indicates that γ-interferon induces pure populations of macrophages to express the smooth muscle contractile protein α-actin, which is not normally expressed by these cells. Thus, it may be possible for macrophages to be a source of cells containing contractile protein under certain inflammatory conditions.
[0142](b) In Vivo Studies
[0143]Definitive proof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com