Methods for Treating Skin Pigmentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Protease Inhibitors Affect Pigmentation
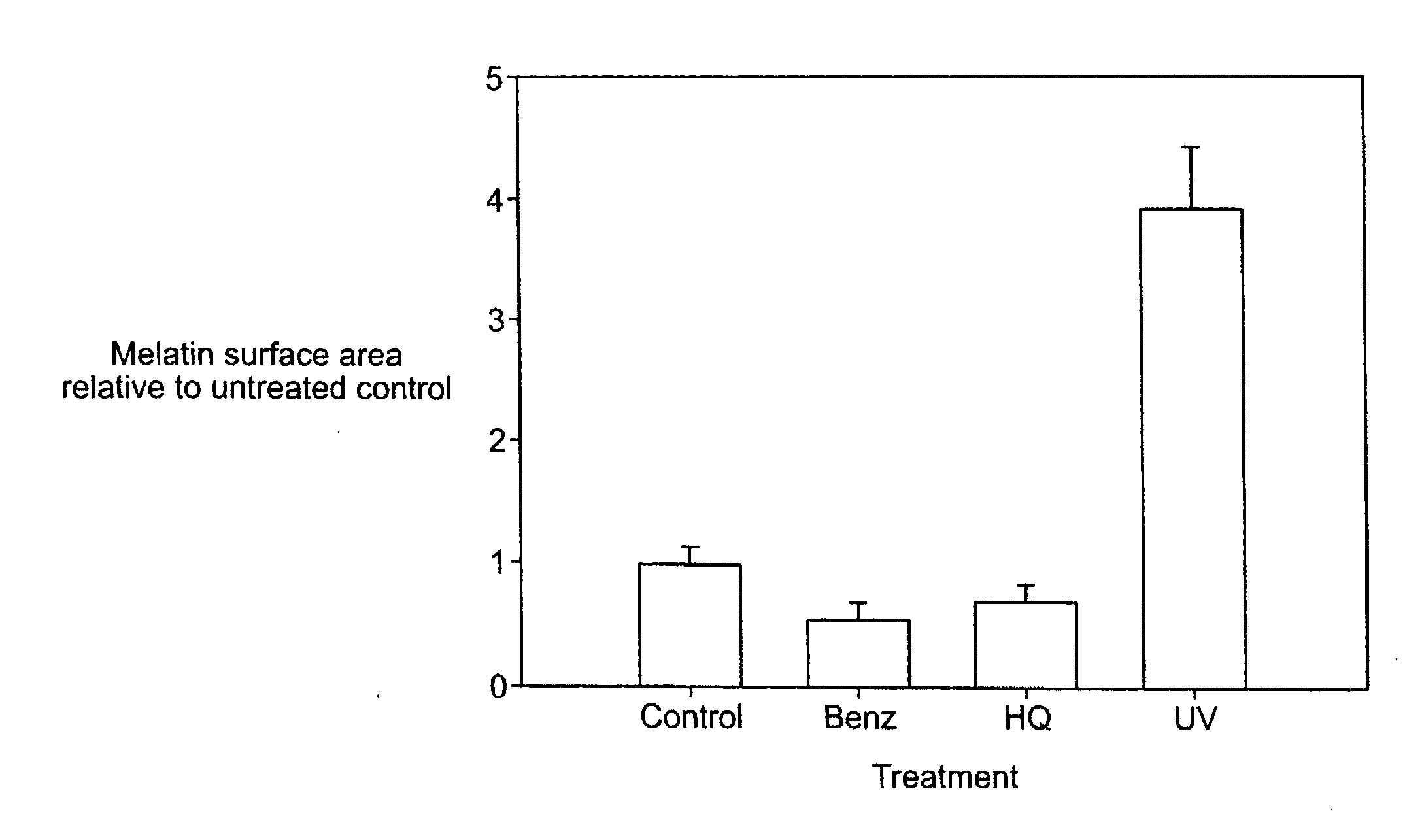
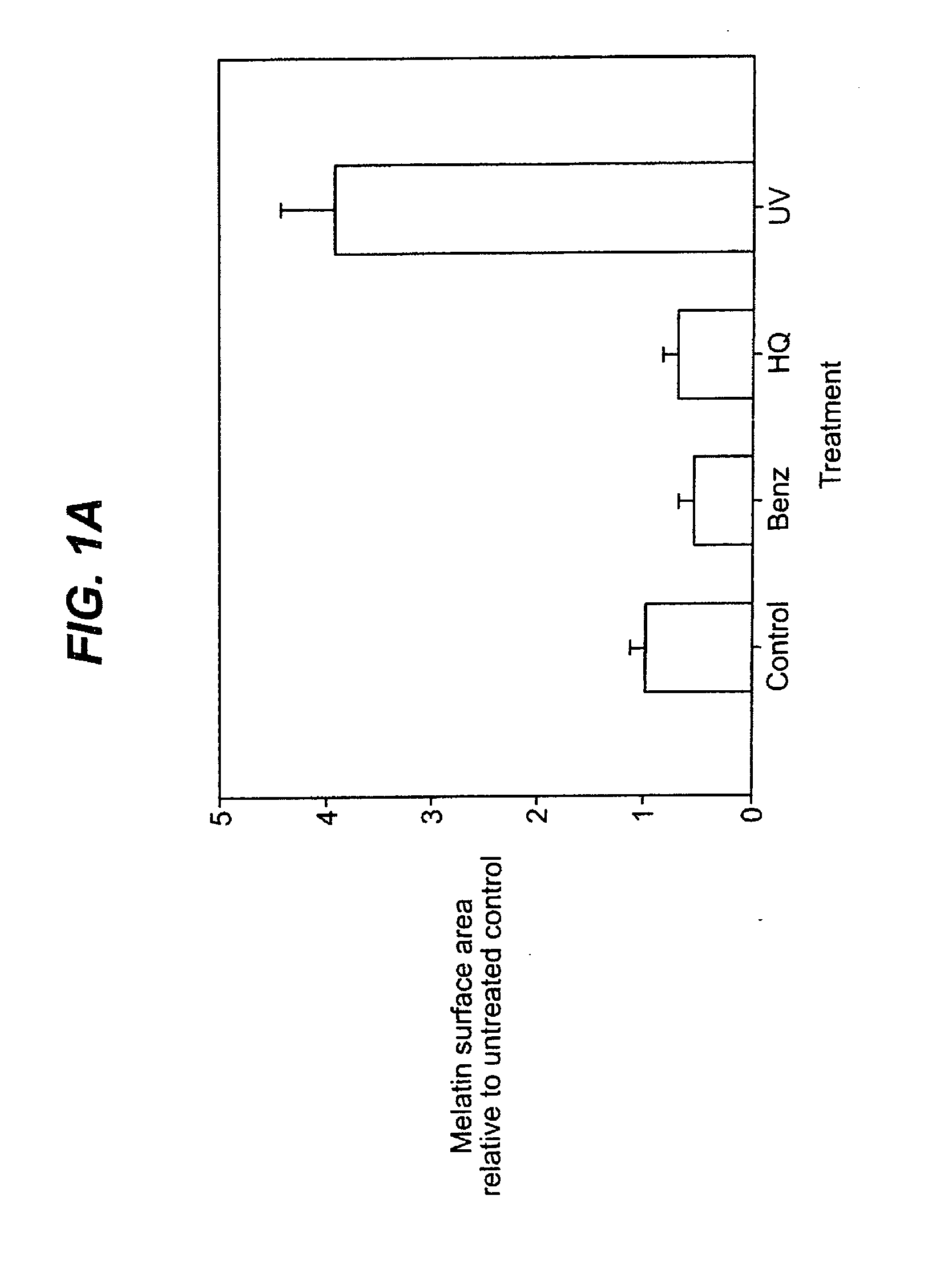
[0049]In order to study the possible roles of the PAR-2 pathway in pigmentation, an in vitro epidermal equivalent system was used. The epidermal equivalent system used contained melanocytes. One epidermal equivalent system which is useful in performing this study is the MelanoDerm system, available commercially from MatTek Co. This system contains human normal melanocytes, together with normal, human-derived epidermal keratinocytes, which have been cultured to form a multi layered, highly differentiated model of the human epidermis. In the following examples, equivalents were treated with test compounds for three days and samples were harvested on the fourth day after beginning of treatment. The harvested equivalents were stained with DOPA (a substrate for tyrosinase) and H&E (a standard histological stain) or with Fontana-Mason (F&M) staining, another stain known to those of skill in the art. F&M staining is a silver staining technique that cl...
example 2
A Protease-Activated Receptor is Involved in Pigmentation
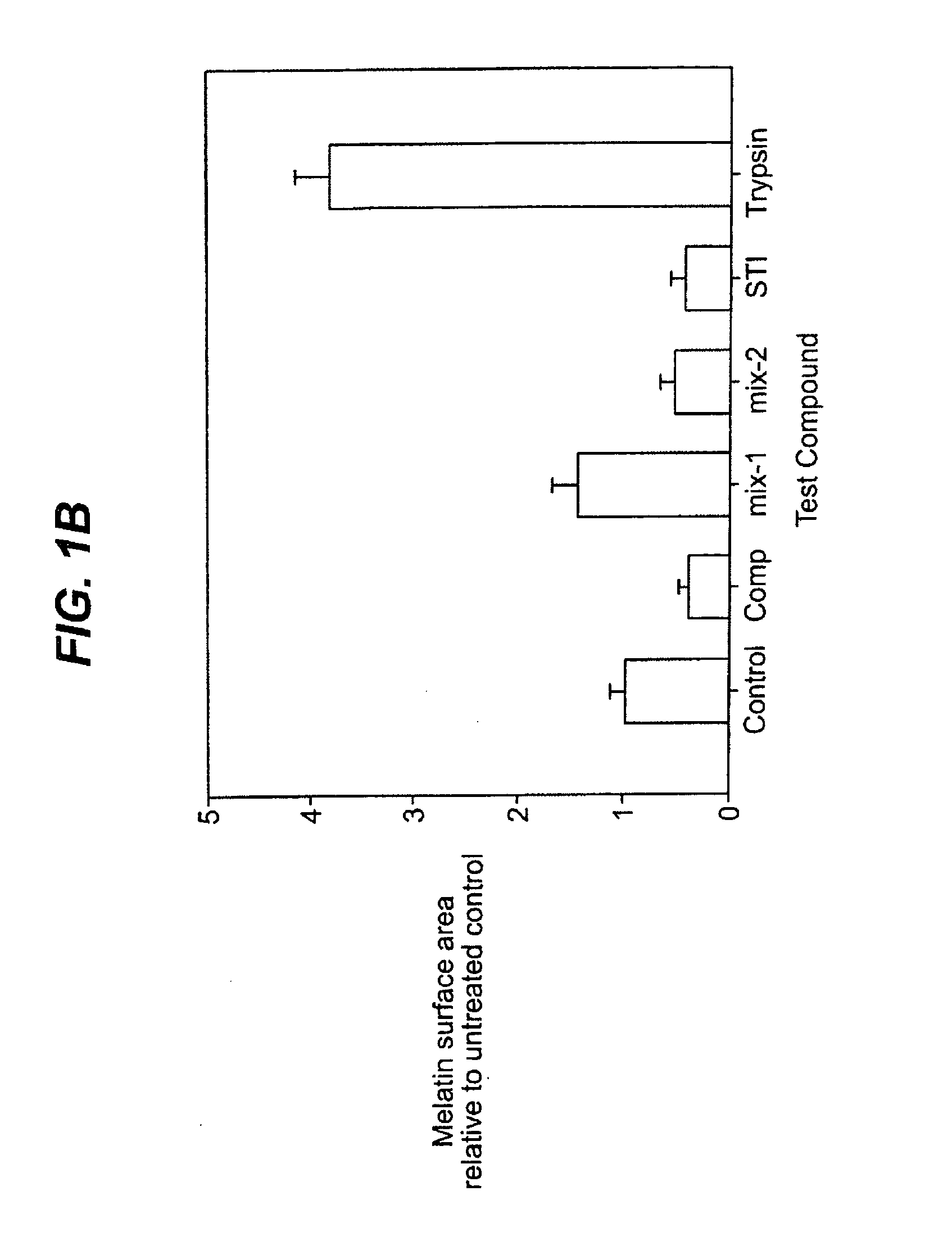
[0055]Example 1 demonstrates that STI reduces pigmentation. STI inhibits trypsin. Because trypsin is known to activate TR and PAR-2, we tested the possible involvement of TR and PAR-2 in pigmentation. MelanoDerm human epidermal equivalents were treated with the TR and PAR-2 agonists and antagonists set forth in Table B below daily for three days. On the fourth day, the samples were harvested, fixed, and DOPA, H&E or F&M staining was performed. Histological and whole-equivalent examination revealed changes in pigmentation following the treatments. FIG. 2 depicts the results of this example. As shown therein, the PAR-2 peptide agonist SLIGRL induced pigmentation in individual melanocytes. Treatment with Compound I, an inhibitor of thrombin and trypsin, resulted in decreased pigmentation.
[0056]FIG. 3 shows the results of the studies set forth in this example, representing the level of pigmentation in MelanoDerm equivalents treate...
example 3
A Dose-Response Relation Between Protease-Activated Receptors Signaling and Melanogenesis
[0057]MelanoDerm equivalents were treated with increasing concentrations of SLIGRL, the PAR-2 peptide agonist, at 0, 10 and 50 μM in the same manner as set forth in Example 2. F&M staining was performed in the fourth day. As shown in FIG. 4A, increasing concentrations of SLIGRL, the PAR-2 activator, result in increased pigmentation. Trypsin, a PAR-2 activator, has the same effect. Treatment with increasing concentrations of Compound I, the thrombin and trypsin inhibitor, from 0.1 pM to 1 μM resulted in decreasing pigmentation (see FIG. 4A). Pretreatment of the equivalents with UVB irradiation increased melanogenesis, relative to untreated controls. Compound I was able to reduce this UVE-induced pigmentation as well (FIG. 4B). This example demonstrates a dose-response relation for increasing and decreasing pigmentation with the modulation of PAR-2 signaling. This example also demonstrates that Co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com