Methods of treating neurological conditions with hematopoeitic growth factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Focal Cerebral Ischemia
Procedure for Inducing Focal Cerebral Ischemia (MCAO, Middle Cerebral Artery Occlusion)
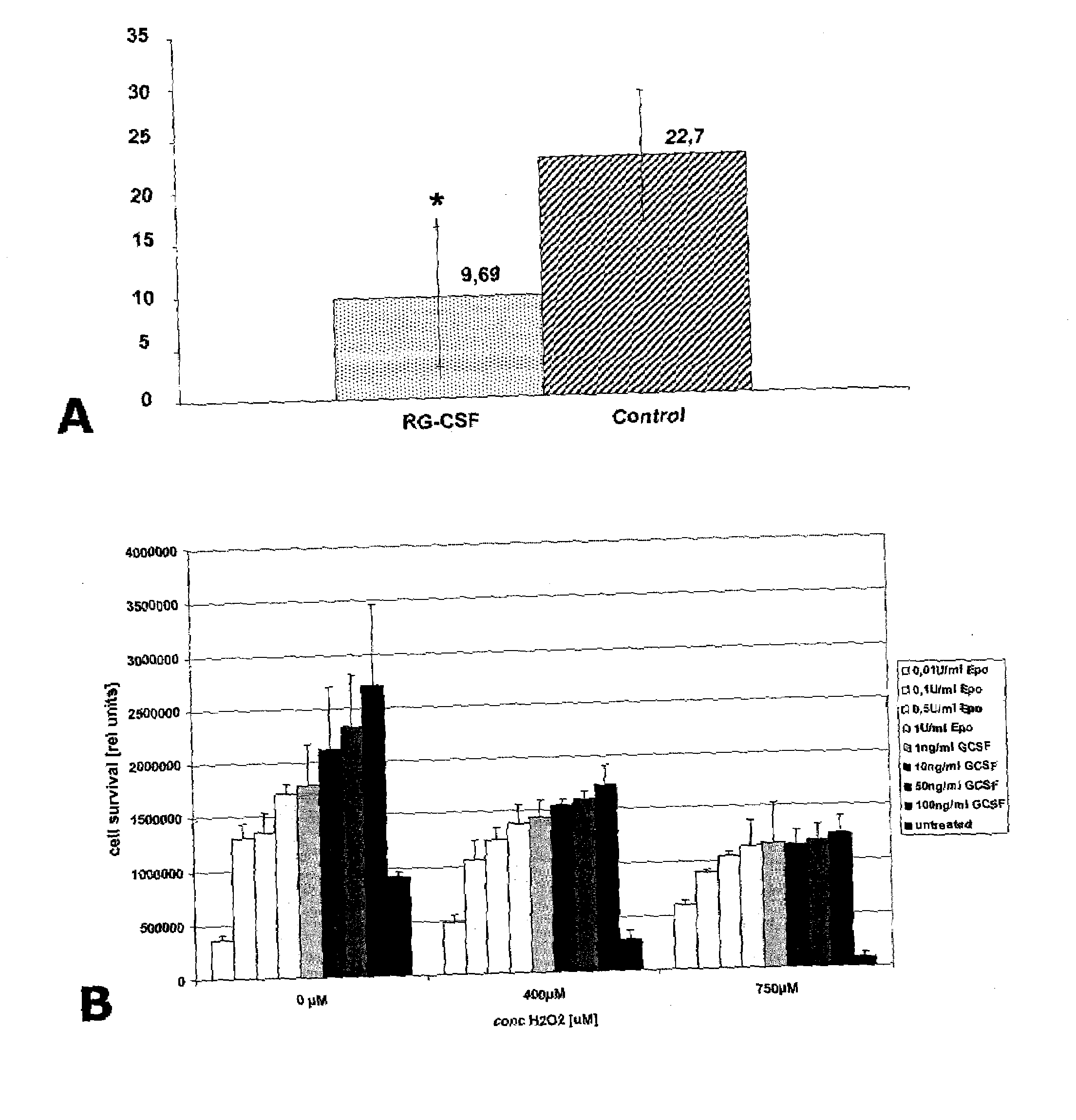
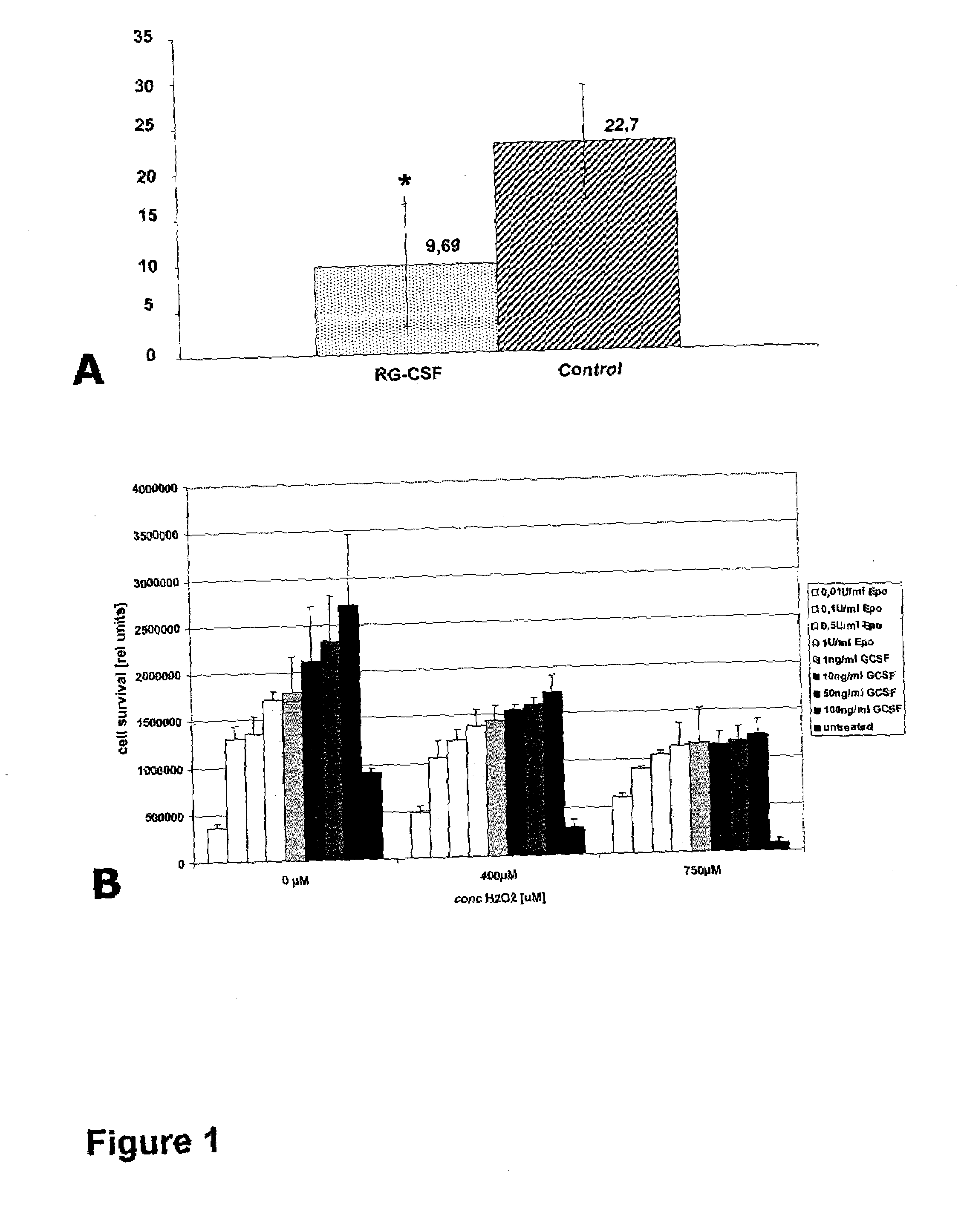
[0310]Experimental protocols were approved by the local ethics committee. Twenty-four male Wistar rats (Charles River, Germany) weighing 280 to 320 g were randomly assigned to the following groups: A (Control group, n=12, ischemia for 90 min, treatment with 2 ml saline 0.9% for 90 min beginning 30 min after vessel occlusion); B (GCSF group, n=12, ischemia for 90 min, treatment with 60 μg / kg body weight of recombinant human GCSF, Neupogen®, Amgen, Europe B.V., Netherlands, soluted in 2 ml saline 0.9% for 90 min beginning 30 min after vessel occlusion. Alternatively, any GCSF or derivative or formulation of other source (another manufacturer (e.g. Lenogastrim™ by Roche or Granocyte™ by Chugai or Albugranin™ by HGS or Neulasta™ by Roche / Amgen) can be used here.
[0311]Animals then were anesthetized with an intraperitoneal injection of 100 mg / kg body weight ketamine hydrochloride ...
example 2
Immunohistochemistry in the Context of Focal Cerebral Ischemia
Immunohistochemical Methods Used
[0317]For morphological analysis of STAT3 activation (FIG. 6) and GCSFR distribution in infarcted brains, and counts of neutrophilic granulocytes, a 2-mm-thick brain slice of GCSF-treated animals and controls was immersion fixed in 4% paraformaldehyde in 0.1 mol / l phosphate buffer for 24 hrs (n=5 per group). After paraffin-embedding, 1-μm-thick sections were cut and used for H&E staining, Nissl staining and immunohistochemical analysis.
[0318]Immunohistochemical studies were performed with antisera against myeloperoxidase (DAKO, Carpinteria, Calif., USA), glial fibrillary acidic protein (GFAP) (DAKO, Carpinteria, CA, USA), GCSFR (Santa Cruz Biotechnology Inc., Santa Cruz, Calif., USA) and STAT3 (Santa Cruz Biotechnology Inc., Santa Cruz, Calif., USA). For antigen retrieval, sections provided for GCSFR and STAT3 immunohistochemistry were heated for 20 min in a 10 mM citrate buffer at 99° C. S...
example 3
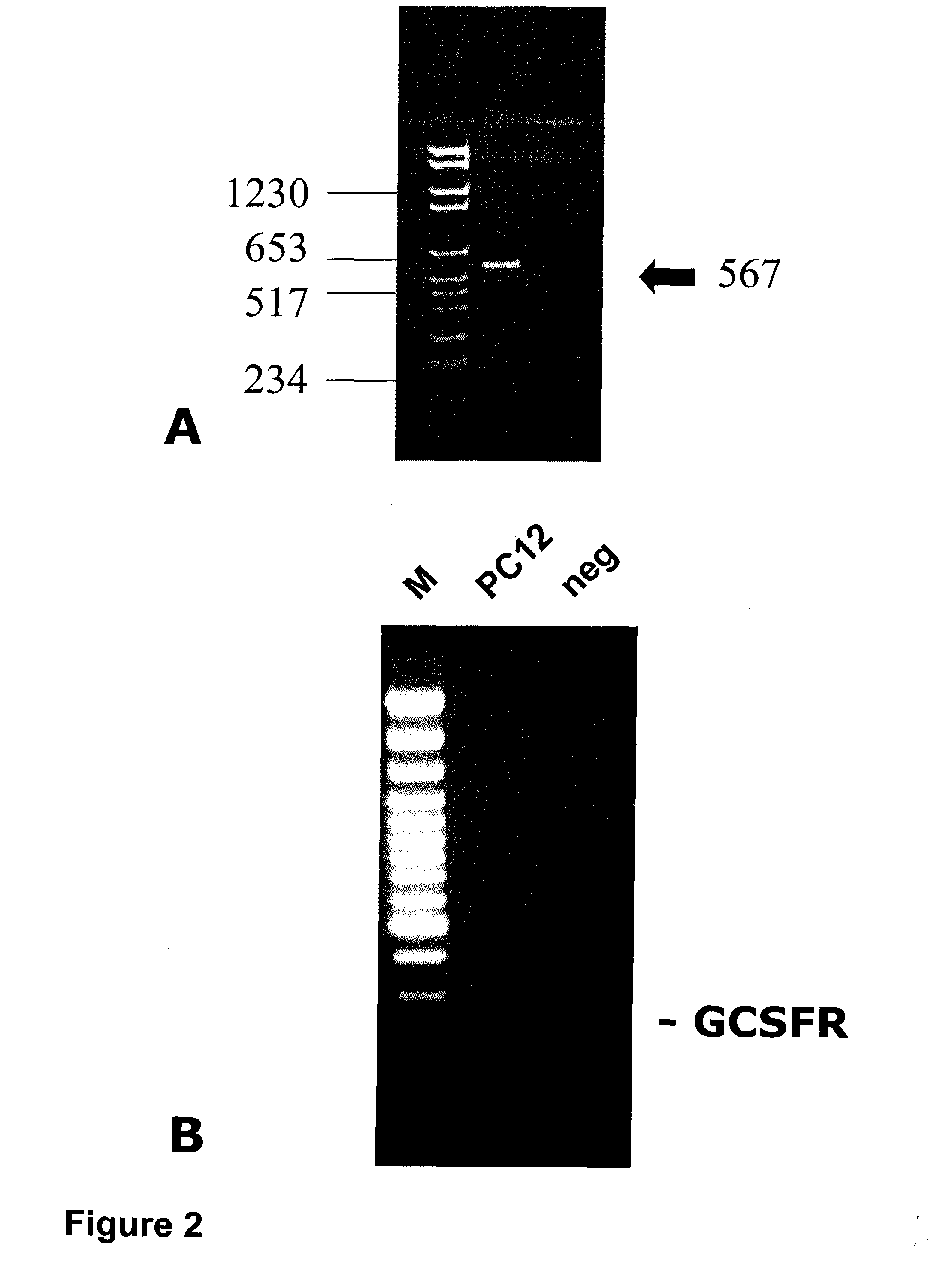
Western Blots and PCR in the Context of Focal Cerebral Ischemia
Western Blots (FIG. 3)
[0324]For immunoblotting, brain tissue (transient ischemia of 2 hours) was lysed in 20 volumes (w / v) of homogenization buffer (320 mM sucrose, 0.1 mM phenylmethylsulfonyl fluoride, and 2 μg / ml Pepstatin) at 4° C. Homogenates were centrifuged at 9,200 G for 15 min at 4° C. After resuspending pellets in 1 / 10 of the homogenization volume, aliquots for protein determination (Bio-Rad protein-assay, Munich, Germany) were separated and samples were rapidly frozen in nitric oxide and stored at −70° C. Per lane 15 μg protein were loaded on a 8% SDS polyacrylamide gel containing 4 M urea and electrophoresed under standard conditions. Proteins were electrophoretically transferred to Immobilon-P™ membranes (Millipore Corp., Eschborn, Germany) by semi-dry blotting. After blocking in 3% nonfat dry milk in TBST (20 mM Tris base, pH 7.6, 137 mM NaCl and 0.05% Tween-20) for 1 hour at room temperature (RT), membranes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com