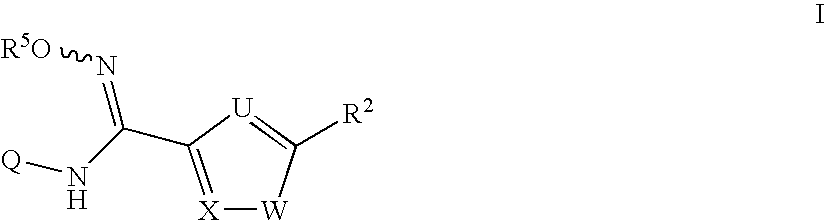
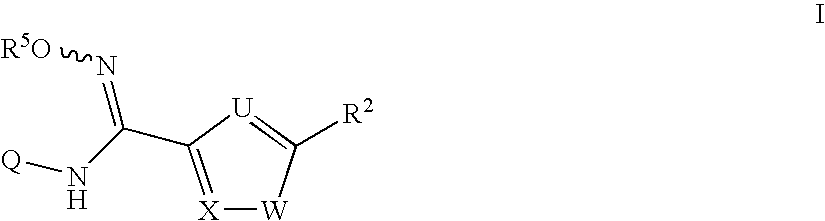
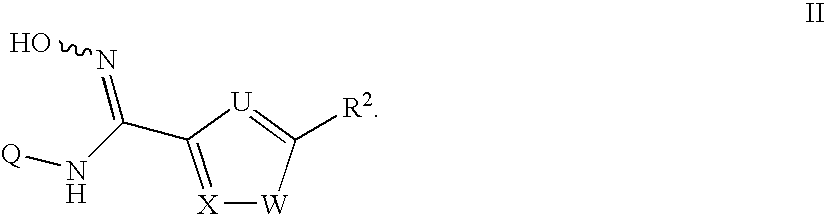N-hydroxyamidinoheterocycles as modulators of indoleamine 2,3-dioxygenase
a technology of indoleamine and hydroxyamidinoheterocycles, which is applied in the direction of biocide, drug composition, immunological disorders, etc., can solve the problems of quinolinic acid (quin), kynurenine metabolites such as 3-hydroxy-kynurenine, and inability of fetus to fully explain the survival of fetal allografts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
N-(3-Chloro-4-fluorophenyl)-N′-hydroxy-5-[2-(methylamino)-1,3-thiazol-4-yl]isoxazole-3-carboximidamide
[0197]
Step A: Ethyl-3-[2-(methylamino)-1,3-thiazol-4-yl]isoxazole-3-carboxylate
[0198]
[0199]A solution of ethyl 3-(bromoacetyl)isoxazole-3-carboxylate (100 mg, 0.40 mmol) and methylthiourea (69 mg, 0.76 mmol) in acetic acid (3.0 mL) was heated at 150° C. in the microwave for 15 minutes. Solvent was removed in vacuo to give the crude which was used without purification. MF=C10H11N3O3S; LCMS calculated for C10H11N3O3S (M+H)+: m / z=254.
Step B: 3-[2-(Methylamino)-1,3-thiazol-4-yl]isoxazole-3-carboxylic acid
[0200]
[0201]A solution of ethyl-3-[2-(methylamino)-1,3-thiazol-4-yl]isoxazole-3-carboxylate (65 mg, 0.26 mmol) in ethanol (3.0 mL) was treated with 1N aqueous sodium hydroxide (0.30 mL, 0.28 mmol) and the mixture was stirred at room temperature for 2 h. The mixture was neutralized with 1 N aqueous hydrochloric acid and concentrated in vacuo to give the crude which was used without purif...
example a
Human idoleamine 2,3-dioxygenasae (IDO) Enzyme Assay
[0211]Human idoleamine 2,3-dioxygenase (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N′-formylkynurenine. The assays were performed at room temperature as described in the literature using 95 nM IDO and 2 mM D-Trp in the presence of 20 mM ascorbate, 5 μM methylene blue and 0.2 mg / mL catalase in 50 mM potassium phosphate buffer (pH 6.5). The initial reaction rates were recorded by continuously following the absorbance increase at 321 nm due to the formation of N′-formlylkynurenine. See: Sono, M., Taniguchi, T., Watanabe, Y., and Hayaishi, O. (1980) J. Biol. Chem. 255, 1339-1345. Compounds of the invention were found to be inhibitors of IDO according to this assay. Data is provided below in Table 2. The symbol “+” indicates IC5050≦10,000 μM. The symbol “+++” indicates IC50>10,000 μM.
TABLE 2Ex....
example b
Determination of Inhibitor Activity in HeLa Cell-Based Indoleamine 2,3-dioxygenase (IDO) / Kynurenine Assay
[0212]HeLa cells (#CCL-2) were obtained from the American Type Tissue Culture Collection (ATCC, Manassas, Va.) and routinely maintained in minimum essential medium (eagle) with 2 mM L-glutamine and Earle's BSS adjusted to contain 1.5 g / L sodium bicarbonate, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate and 10% fetal bovine serum (all from Invitrogen). Cells were kept at 37° C. in a humidified incubator supplied with 5% CO2. The assay was performed as follows: HeLa cells were seeded in a 96 well culture plate at a density of 5×103 per well and grown overnight. On the next day, IFN-γ (50 ng / mL final concentration) and serial dilutions of compounds (in total volume of 200 μL culture medium) were added into cells. After 48 hours of incubation, 140 μL of the supernatant per well was transferred to a new 96 well plate. 10 μL of 6.1 N trichloroacetic acid (#T0699, Sigma) was mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com