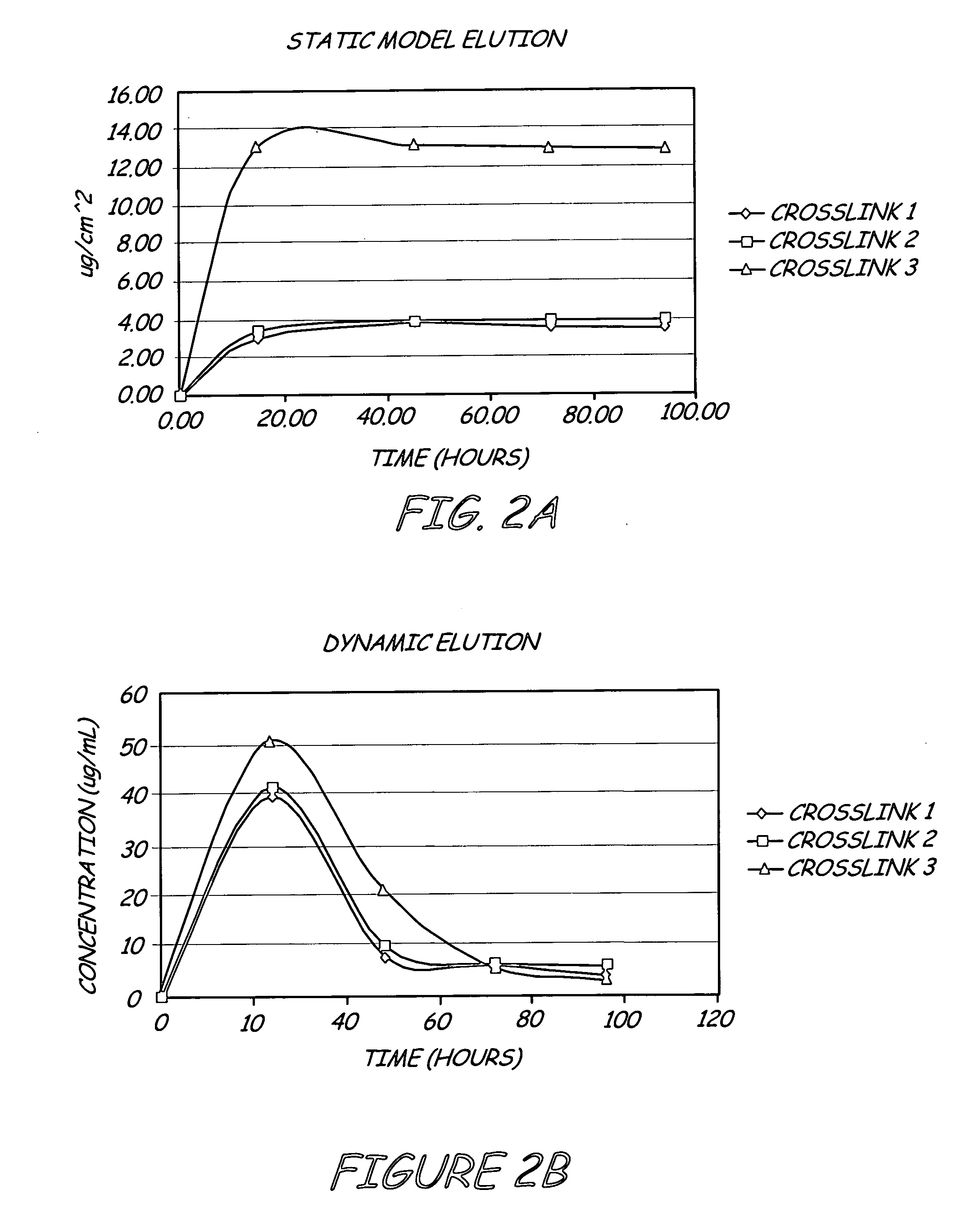
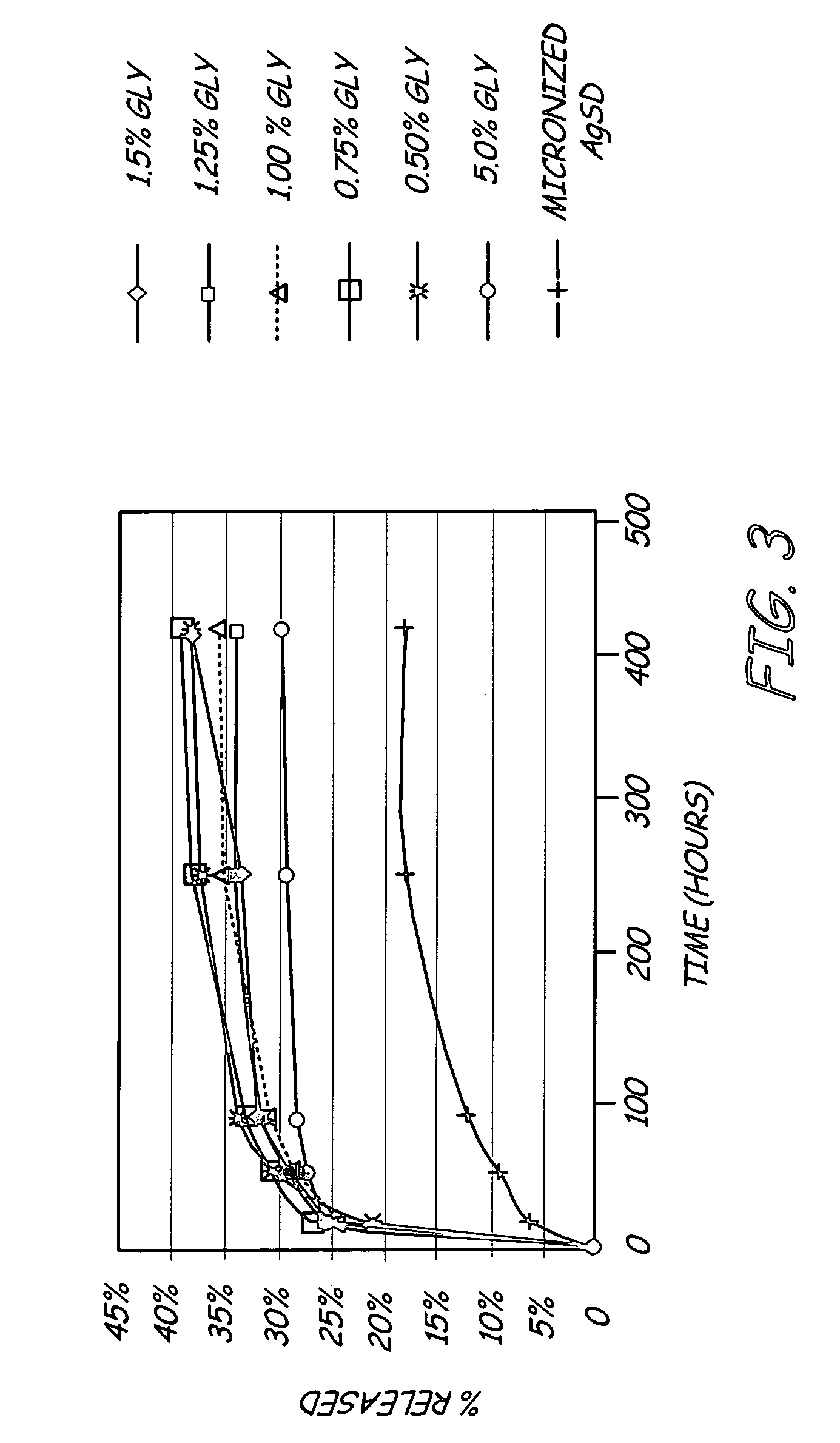
Antimicrobial coating for inhibition of bacterial adhesion and biofilm formation
a technology of antimicrobial coating and biofilm, which is applied in the direction of biocides, paints, prostheses, etc., can solve the problems of affecting the integrity of medical devices, forming biofilms on the surface of medical devices, and serious patient problems, so as to increase the antimicrobial effect of coating and inhibit bacterial adhesion and biofilm formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Coating Formulation Containing Solubilized Silver Sulfadiazine (AgSD)
[0053]A coating formulation comprising AgSD (20 g / L) was prepared as follows. Nitric acid (64 mL, 70%) was added to 800 mL H2O. The resulting nitric acid solution was then heated to 70° C. using a double boiler. AgSD (20 g) was added to the nitric acid solution with stirring using and overhead stirrer with a dissolving stirring shaft. The AgSD was dissolved in a couple minutes. The final volume of the AgSD solution was brought to 1.0 L with H2O.
[0054]Additional coating ingredients may be added when the AgSD (20 g / L) coating formulation is complete. Higher concentrations of AgSD such as 30 g / L may be prepared using analogous procedures.
example 2
Coating Formulation Preparation
[0055]A liter of coating formulation comprising AgSD (20.0 g) and PVA (50.0 g, MW=124,000 to 186,000, 87-89% hydrolysis) was prepared as follows.
[0056]In an appropriate sized temperature controlled mixing vessel set at moderate mixing, Nitric acid (64 mL, 70%) was added to purified H2O and diluted to 800 mL. The temperature of the circulating heater with oil & pump was set between 65° C. and 70° C. The variable speed overhead mixer with dissolving stirrer attachment was set at 500 rpm. AgSD 20.0 g was added slowly to the mixing water and acid mixture. The solution was mixed for a minimum of 15 minutes. The dissolution was confirmed by turning off the mixer and observing that no solid particles settle out after 60 seconds. The temperature of the circulating heater was set to 80° C. and the stirrer was turned back on. The temperature in temperature controlled vessel containing the drug / acid mixture was allowed to reach at least 75° C. before proceeding.
[...
example 3
Coating Formulation Preparation with TiO2
[0059]A liter of coating formulation comprising AgSD (20.0 g), PVA (50.0 g, MW=124,000 to 186,000, 87-89% hydrolysis) and TiO2 (2.0 g) was prepared as follows.
[0060]In an appropriate sized temperature controlled mixing vessel set at moderate mixing, Nitric acid (64 mL, 70%) was added to purified H2O and diluted to 800 mL. The temperature of the circulating heater with oil & pump was set between 65° C. and 70° C. The variable speed overhead mixer with dissolving stirrer attachment was set at 500 rpm. AgSD 20.0 g was added slowly to the mixing water and acid mixture. The solution was mixed for a minimum of 15 minutes. The dissolution was confirmed by turning off the mixer and observing that no solid particles settle out after 60 seconds. The temperature of the circulating heater was set to 80° C. and the stirrer was turned back on. The temperature in temperature controlled vessel containing the drug / acid mixture was allowed to reach at least ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com