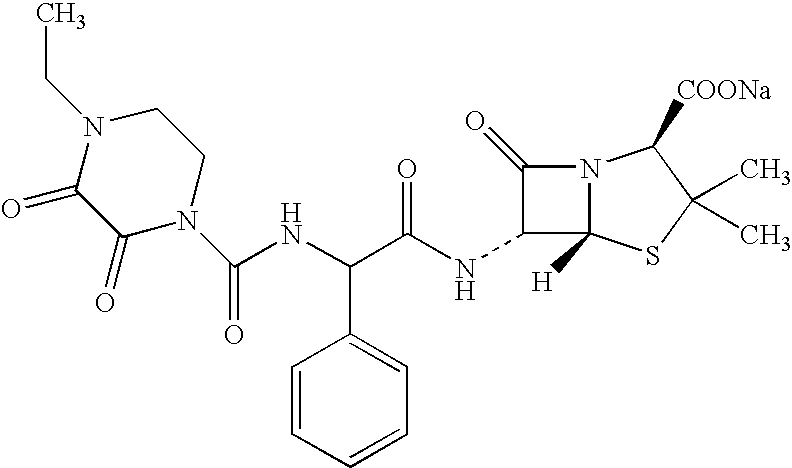
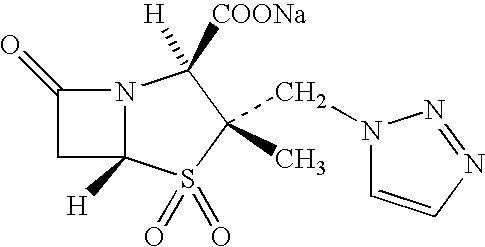
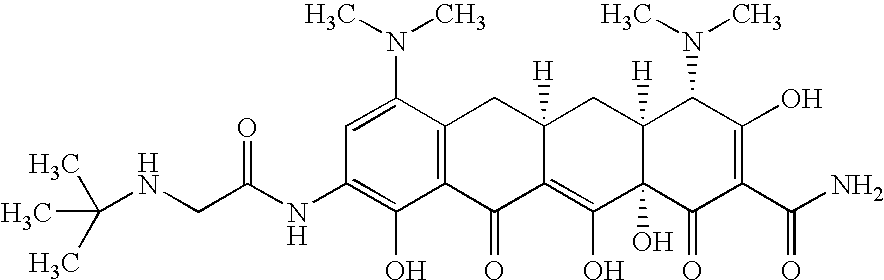
Treating cystic fibrosis with antibiotics via an aerosol drug
an aerosol drug and cystic fibrosis technology, applied in the directions of aerosol delivery, dispersed delivery, heterocyclic compound active ingredients, etc., can solve the problem that the system has not been applied to the delivery of antibiotics to the lungs to treat cystic fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
Tygacil®(Tigecycline) for Deep Lung Delivery
[0029] The commercial Tygacil®2nd Generation product was used to conduct the study. Sterile Water for injection and 0.9% Normal Saline were used at diluents. Tygacil® is a sterile, lyophilized powder for intravenous infusion, containing 53 mg of the Tigecycline active ingredient. Tygacil® additionally contains lactose monohydrate as a diluent / stabilizer and hydrochloric acid and / or sodium hydroxide (as needed) for pH adjustment. The product is supplied in a single dose; Type I, clear, glass vial, sealed under a blanket of nitrogen with a gray butyl rubber stopper and a snap-off aluminum crimp seal.
The Quantitative Composition of Tygacil® is Depicted in Table 1 Below.
[0030]
TABLE 1Quantitative Composition for Tygacil ®Reference toIngredientStandardsFunctionQuantity per VialTigecyclineaIn-HouseActive 53 mgMonographLactoseNF / Ph. Eur.bDiluent / 106 mgMonohydrateStabilizerHydrochloric AcidNF / Ph. Eur.pH AdjustmentQ.S. to adjust pHSodium Hydroxid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com