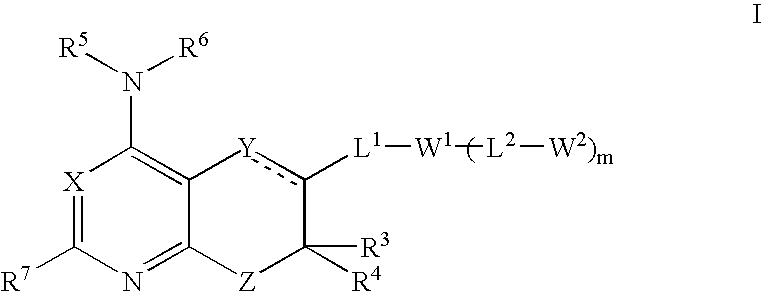
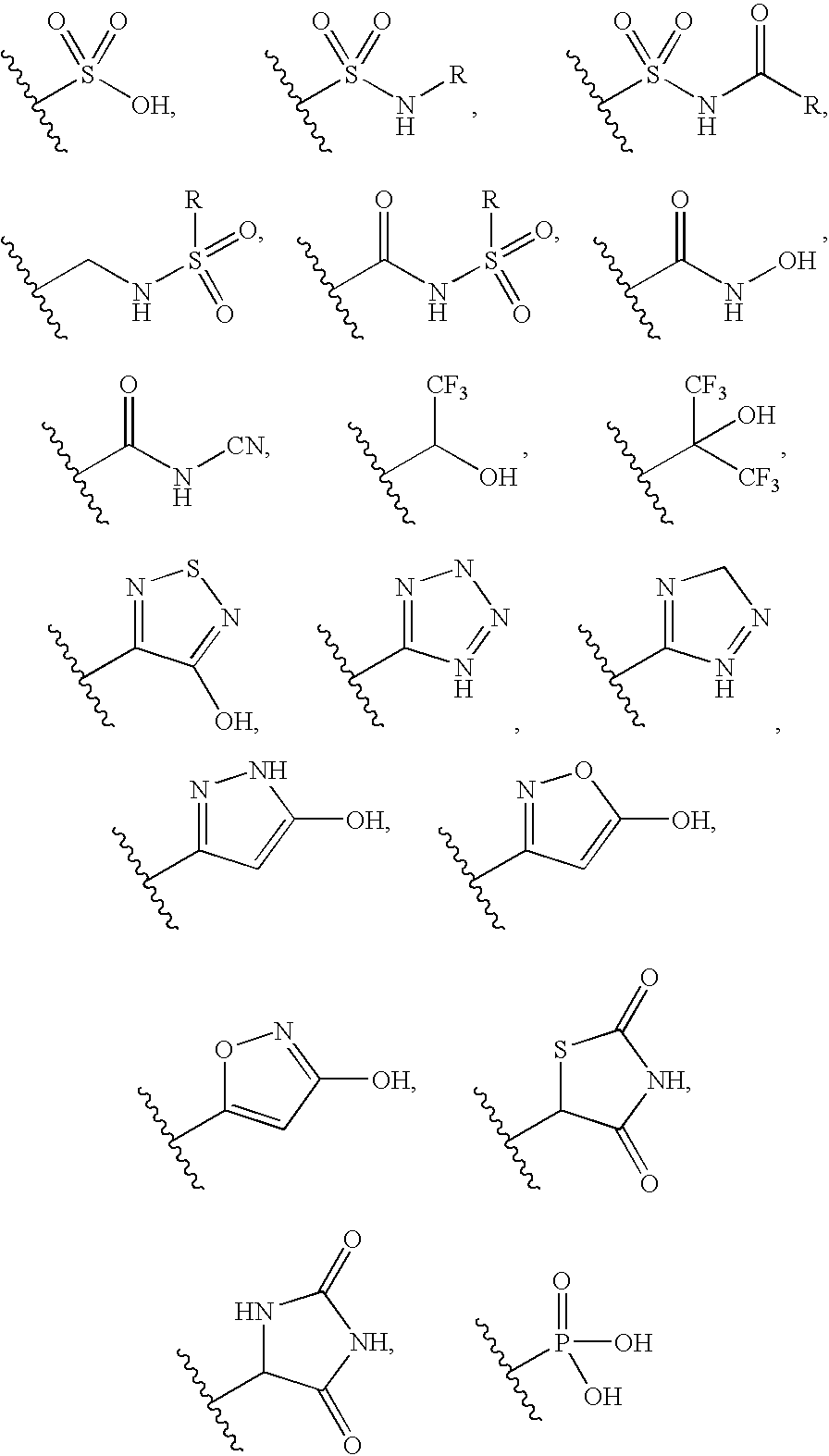
Fused Bicyclic Nitrogen-Containing Heterocycles
a technology of bicyclic nitrogen and heterocycles, which is applied in the field of fused bicyclic nitrogen-containing heterocycles, can solve the problems of often adverse effects of hypertriglyceridemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0129] This example illustrates the preparation of the compound designated Ex. 1.
[0130] To a suspension of 4,5-diamino-6-hydroxypyrimidine (1.0 g, 7.93 mmol) and 4-bromophenacyl bromide (2.2 g, 7.93 mmol) in EtOH (20 mL) was added NaHCO3 (666 mg, 7.93 mmol), and the mixture was stirred for 1.5 h at 80° C. After cooling, the reaction mixture was concentrated, and the residue was diluted with CHCl3 (40 mL). After filtration of insoluble material, the filtrate was concentrated. The residue was triturated with toluene to give the desired compound Ex. 1 (840 mg) as a pale yellow crystal, m.p.: >200° C. IR (cm−1): 3291, 3145, 1634, 1586. MS (ESI+): 305, 307 (100). 1H NMR(DMSO-d6, 400 MHz): 5.44 (s, 2H), 7.10 (br s, 2H), 7.69 (d, 2H, J=8.6 Hz), 7.93 (s, 1H), 8.03 (d, 2H, J=8.6 Hz).
example 1-2 to 1-37
[0131] The compounds shown in Table 1 were obtained in the same manner as in Example 1.
example 2
[0132] This example illustrates the preparation of the compound designated Ex. 2.
[0133] a)
[0134] At 0° C., to a solution of triethyl phosphonoacetate (2.6 mL, 12.91 mmol) in DMF (5.5 mL), sodium hydride (60% in oil, 517 mg, 12.91 mmol) was added portionwise, and the reaction mixture was stirred at room temperature for 30 min. A solution of 4-phenylcyclohexanone in DMF (2.0 mL) was added. After stirring for 0.5 h, the mixture was poured into 5% aq. KHSO4 (10 mL) and extracted with diethyl ether (10 mL). The organic layer was successively washed with water (5 mL) and brine (5 mL), dried over MgSO4 and concentrated. The residue was purified by column chromatography (hexane / AcOEt=7 / 1) to give compound 2 (2.0 g) as colorless oil.
[0135] b)
[0136] To a stirred solution of 2 (500 mg, 2.05 mmol) in EtOH (5 mL) was added 10% Pd / C (50 mg). The mixture was stirred at room temperature for 1 h under an atmospheric pressure of hydrogen. The catalyst was removed by filtration, and the filtrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com