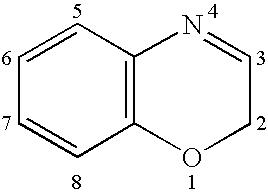
Androgen receptor modulator compounds and methods
a technology of androgen receptors and modulators, applied in the field of nonsteroidal compounds, can solve the problems of modulators of steroid receptors and homeostatic imbalances in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1,2,3,6-Tetrahydro-1-methyl-9-(trifluoromethyl)-7H-[1,4]oxazino[3,2-g]quinolin-7-one (Compound 101, Structure 6 of Scheme I, where R1═H, R=trifluoromethyl, R6═H, Rx═H)
[0154] General Method 1: Cyclization of an α-chloroacetyl chloride to 2-amino-5-nitrophenol. To a solution of 2-amino-5-nitrophenol (1.0 equiv), NaHCO3 (2.4 equiv) in 4-methyl-2-pentanone (0.6 mL / mmol) and water (0.6 mL / mmol) was added an α-chloroacetyl chloride derivative (1.15equiv) via syringe pump over 45 min at 0° C. The reaction mixture was allowed to warm to room temperature and then refluxed overnight. The crude reaction mixture was allowed to cool to room temperature, filtered and washed with water (3×1.2 mL / mmol) to afford the desired product as a tan solid.
[0155] 7-Nitro-2H-1,4-benzoxazin-3(4H)-one (Structure 2 of Scheme I, where R6═H). This compound was prepared by General Method 1 from 2-amino-5-nitrophenol (6.0 g, 39 mmol), NaHCO3 (7.8 g, 93 mmol), and chloroacetyl chloride (3.58 mL, 45 mmol) to afford ...
example 2
1,2,3,6-Tetrahydro-1,6-dimethyl-9-(trifluoromethyl)-7H-[1,4]oxazino[3,2-g]quinolin-7-one (Compound 102, Structure 7 of Scheme I, where R1═H, R2=trifluoromethyl, R6═H, Rx═H)
[0164] General Method 6: N-Methylation of a pyridone (compounds of Structure 6) to form a compound of Structure 7. To an oven dried rb flask containing a pyridone of Structure 6 (1.0 equiv) in THF (5 mL / mmol) was added portionwise sodium hydride (60% dispersion in mineral oil, 1.2 equiv) under N2. After 30 min, iodomethane (1.2 equiv) was added and the mixture was allowed to stir under N2 an additional 8-10 hrs. The reaction mixture was then diluted with pH 7 phosphate buffer (50 mL / mmol), extracted with CH2Cl2 (3×30 mL) and washed with brine (50 mL / mmol). The organic solution was dried (MgSO4) and concentrated under reduced pressure. Purification by flash chromatography (silica gel, 20:1, CH2Cl2:MeOH) afforded the desired product as a fluorescent-yellow solid.
[0165] 1,2,3,6-Tetrahydro-1,6-dimethyl-9-(trifluorom...
example 3
1-Ethyl-1,2,3,6-tetrahydro-9-(trifluoromethyl)-7H-[1,4]oxazino[3,2-g]quinolin-7-one (Compound 103, Structure 6 of Scheme I, where R1═H, R2=trifluoromethyl, R6═H, Rx═CH3)
[0166] 4-Ethyl-3,4-dihydro-7-nitro-2H-1,4-benzoxazine (Structure 4 of Scheme I, where R6═H, Rx═CH3). This compound was prepared by General Method 3 (EXAMPLE 1) from 3,4-dihydro-7-nitro-2H-1,4-benzoxazine (EXAMPLE 1) (1.15 g, 6.39 mmol), acetaldehyde (3.59 mL, 64.2 mmol) and NaBH3CN (1.95 g, 31 mmol) to afford 984 mg (74%) of 4-ethyl-3,4-dihydro-7-nitro-2H-1,4-benzoxazine, a yellow solid. Data for 4-ethyl-3,4-dihydro-7-nitro-2H-1,4-benzoxazine: Rf0.85 (11.5:1 CH2Cl2:MeOH); 1H NMR (400 MHz, CDCl3) δ 7.81 (dd, 1H, J=9.6,2.6), 7.66 (d, 1H, J=2.7), 6.29 (d, 1H, J=9.2), 4.23 (t, 2H, J=4.7), 3.47 (t, 2H, J=4.7), 3.45 (q, 2H, J=7.2), 1.22 (t, 3H, J=7.0).
[0167] 7-Amino-4-ethyl-3,4-dihydro-2H-1,4-benzoxazine (Structure 5 of Scheme I, where R6═H, Rx═CH). This compound was prepared by General Method 4 (EXAMPLE 1) from 4-ethyl-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com