Application of lipid vehicles and use for drug delivery
a technology of lipid vehicles and lipids, applied in the direction of biocide, drug composition, antibody medical ingredients, etc., can solve the problems of increasing the risk of drug resistance, so as to reduce or prevent antibody-mediated resistance to antigenic therapeutic agents, the effect of easing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
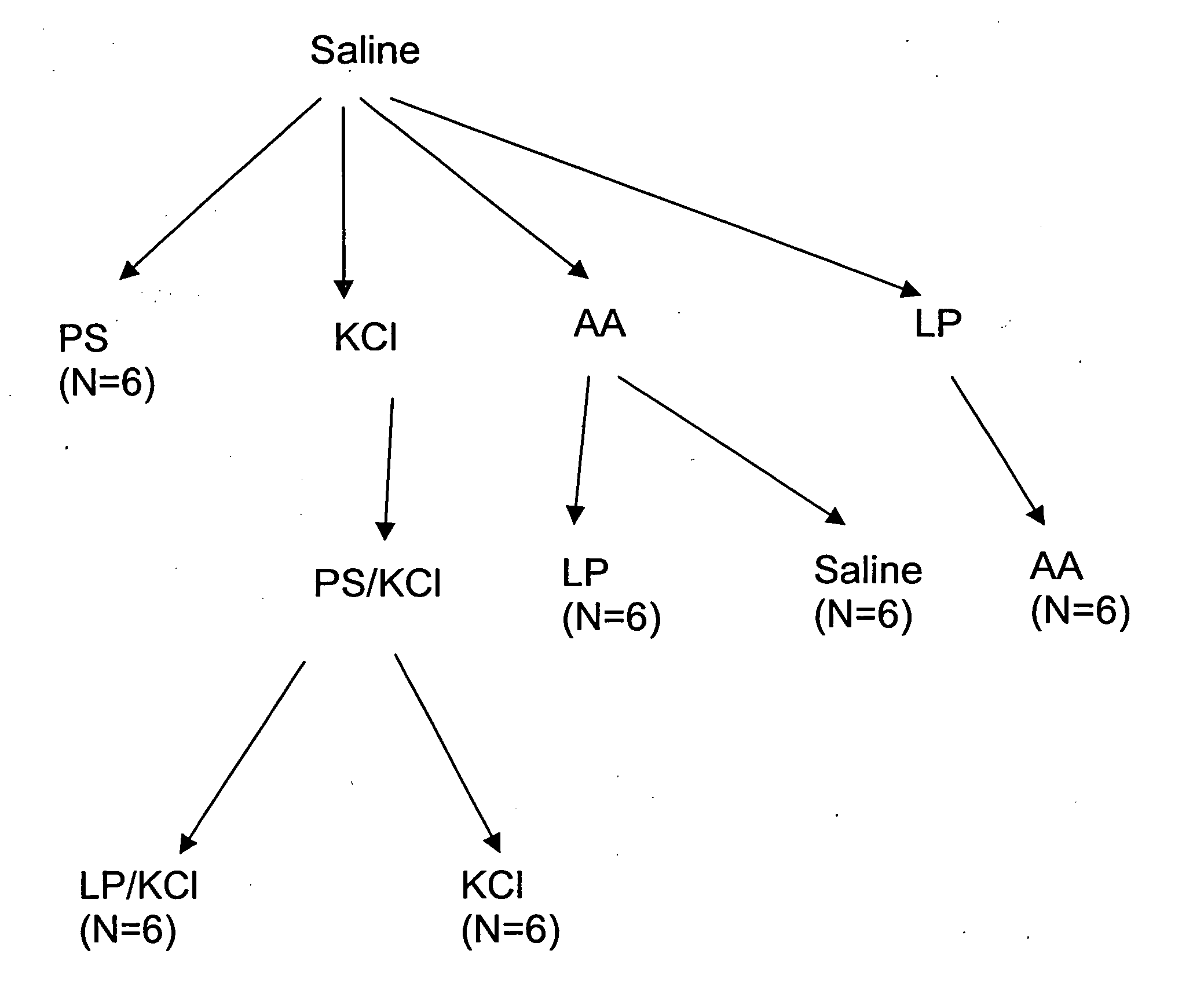
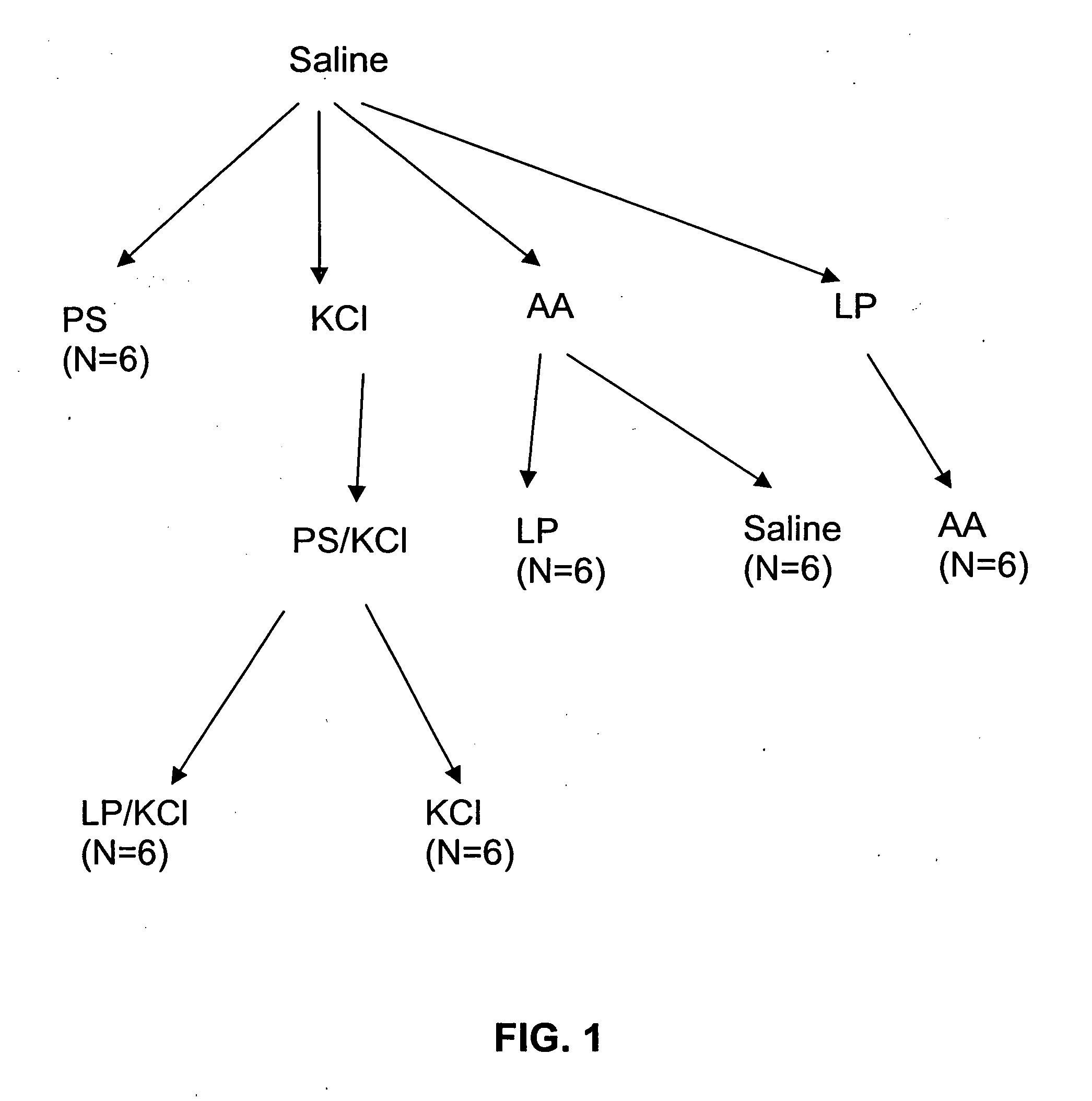
[0147] A hyperactive bladder model in Sprague-Dawley rats was established by exposure to acetic acid, or protamine sulfate (PS) in potassium chloride (KCl) solution. This was followed by instillation of LP (liposomes) in saline (in the case of the former) or LP / KCl. CMG (continuous cystometrogram) changes were examined and results were compared with control (saline instillation), hyperactive bladder (acetic acid or PS / KCl) and treatment with LP.
[0148] Materials and Methods
[0149] Intravesical bladder pressure was recorded via a transurethral catheter in adult female Sprague-Dawley rats anesthetized with urethane (1.2 g / kg) administered by subcutaneous injection (sc). Some animals were pretreated with capsaicin (125 mg / kg, sc) four days prior to the experiments. Continuous cystometrograms (CMGs) were performed by slowly filling the bladder (0.04 ml / min) with solutions of various composition including saline, acetic acid (0.1%), potassium chloride (KCl, 500 mM), protamine sulfate (PS...
example 2
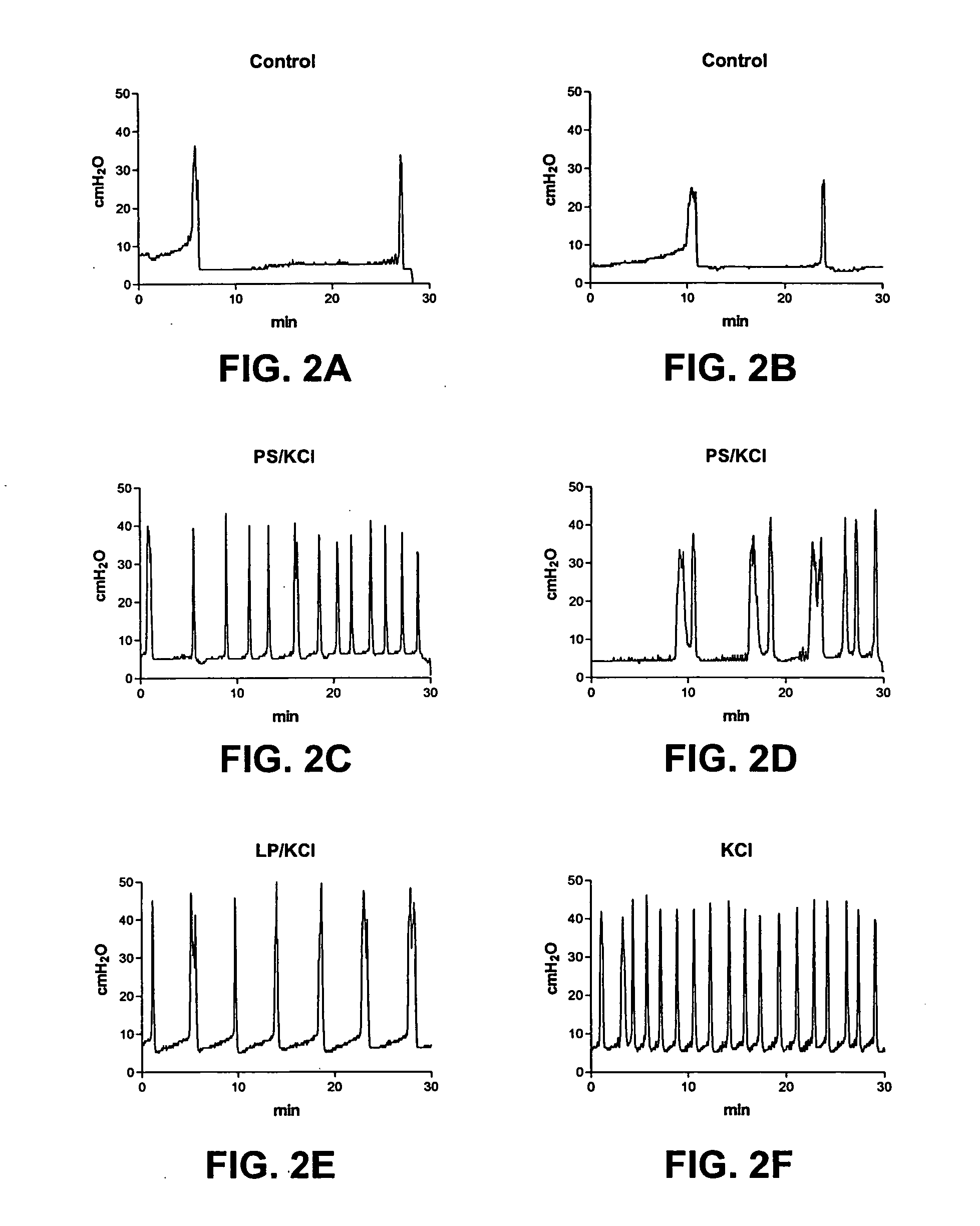
[0160] The results of the experiments described in Example 1 are presented herein below. In summary, ICl (intercontraction interval) was decreased after exposure to acetic acid (79.8% decrease) or PS / KCl (81% decrease). However, ICl was not changed by LP, PS, or KCl alone. The decrease in ICl was partly reversed after infusion of LP (172.8% increase) or LP / KCl (63% increase), but was not significantly changed after by saline or KCl administration. Pretreatment with capsaicin delayed the onset of the irritative effects of acetic acid by approximately 30 to 60 min, but did not change the magnitude after two hours of infusion.
[0161] CMGs in PS / KCl Infusion Group
[0162] As shown in Tables 1A-1C, infusion of PS (10 mg / ml) or KCl (500 mM) alone did not significantly change the CMGs. However infusion of PS / KCl provided an irritative effect after a delay of 30 to 40 min (FIGS. 2B, 2D). The ICl and compliance were significantly reduced by 79-83% (from 15.8±1.4 to 2.7±1.0 min or from 16.3±1....
example 3
[0177] An animal model for acute hyperactive bladder in rats was developed using intravesical infusion of protamine sulfate (PS), an agent used to break down urothelial barrier function, and physiological concentrations of potassium chloride (KCl).
[0178] Materials and Methods
[0179] Continuous CMGs were performed in urethane-anesthetized female rats. The bladder was filled (0.04 ml / min) with normal saline followed by intravesical infusion for a 60 minute period with a test solution comprising either KCl (100 or 500 mM) or PS (10 or 30 mg / ml). Following this, 10 mg / ml PS treated animals were infused intravesically with 100, 300, or 500 mM KCl. Some animals were pretreated with capsaicin (125 mg / ml, sc) four days before the experiments.
[0180] Animal Preparation
[0181] The study was performed on 40 female Sprague-Dawley rats weighing 250-300 gm. Animals were anesthetized with 1.2 gm / kg urethane injected subcutaneously. Body temperature was maintained in the physiological range using ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com