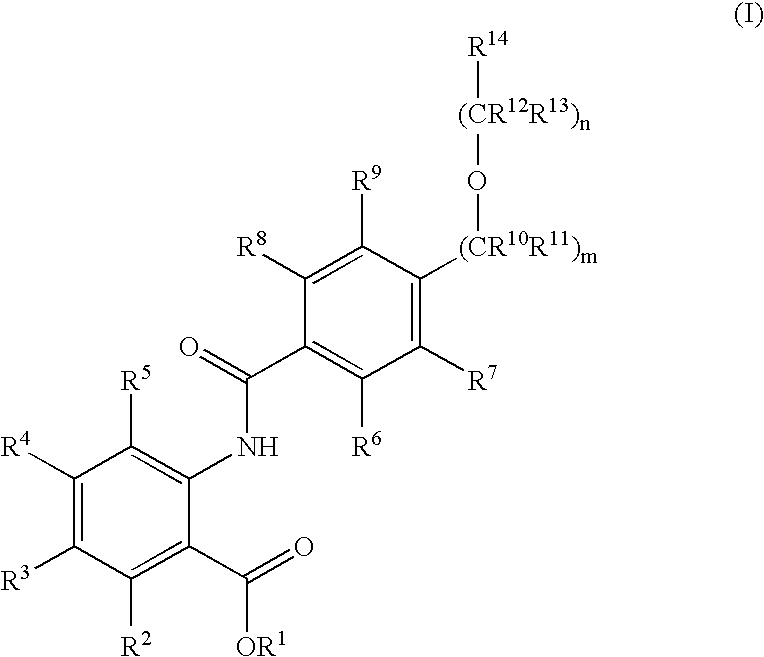
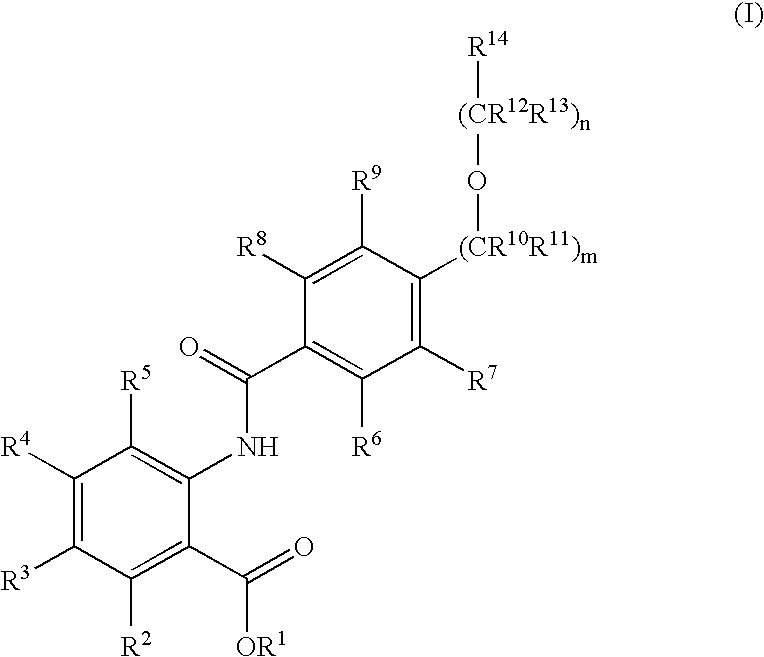
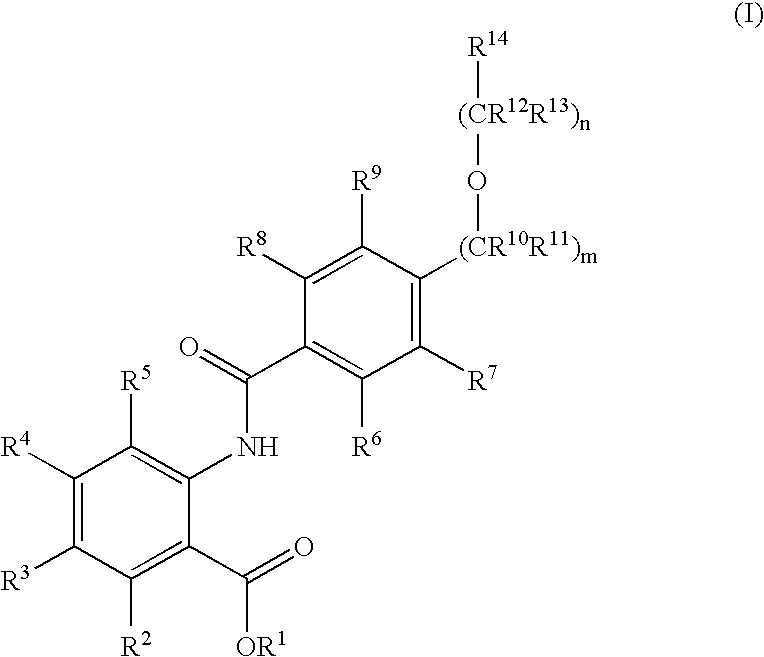
Novel anthranilic acid derivatives
an anthranilic acid and derivative technology, applied in the field of new anthranilic acid derivatives, can solve the problems of reducing plaque stability, increasing cardiovascular risk, and hampered extensive use of niacin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
2-(4-Benzyloxy-benzoylamino)-benzoic acid
Step 1:
2-(4-Benzyloxy-benzoylamino)-benzoic acid methyl ester
[0353] To 2-amino-benzoic acid methyl ester (0.51 mL) and triethylamine (0.69 mL) in dichloromethane (31 mL) at −50° C. was slowly added a solution of 4-benzyloxy-benzoyl chloride [1486-50-6](1 g) in 31 mL of dichloromethane. The reaction mixture was then allowed to warm up to room temperature and stirred for an additional hour. After such time, the reaction mixture was washed with water. The aqueous phase was further extracted with dichloromethane. The combined organic phases were dried over sodium sulfate and concentrated in vacuo. The residue was purified by column chromatography (heptane-ethyl acetate: 0-50%) to yield 2-(4-benzyloxy-benzoylamino)-benzoic acid methyl ester (400 mg). MS (m / e): 362.5 (M+H+, 100%).
Step 2:
2-(4-Benzyloxy-benzoylamino)-benzoic acid
[0354] To 2-(4-benzyloxy-benzoylamino)-benzoic acid methyl ester (50 mg) in methanol (3 mL) was added lithium hydro...
example 2
2-[4-(4-Fluoro-phenoxy)-benzoylamino]-benzoic acid
[0355] To 2-(4-iodo-benzoylamino)-benzoic (acid methyl ester [75541-84-3](50 mg) in 1-methyl-2-pyrrolidinone (1 mL) was added 4-fluorophenol (29.4 mg), copper (I) chloride (6.6 mg), cesium carbonate (85 mg) and 8-hydroxy-chinolin (4.7 mg). The reaction mixture was stirred at 120° C. for 18 hours. After such time, the reaction mixture was allowed to cool down to room temperature, diluted with water, formic acid was added (0.3 mL) and the solution was purified by preparative HPLC to yield 2-[4-(4-fluoro-phenoxy)-benzoylamino]-benzoic acid (49.1 mg). MS (m / e): 332.1 (M−H−, 100%).
example 3
2-[4-(3,4-Dichloro-phenoxy)-benzoylamino]-benzoic acid
[0356] In analogy to example 2, 2-[4-(3,4-Dichloro-phenoxy)-benzoylamino]-benzoic acid was prepared from 2-(4-iodo-benzoylamino)-benzoic acid methyl ester [75541-84-3]and 3,4-dichlorophenol. MS (m / e): 400.0 (M−H−, 100%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com