Therapeutic uses of BPI protein products in BPI-deficient humans
a technology of bpi and protein products, which is applied in the field of newborns, can solve the problems of not explaining the decreased phagocytic and bactericidal activity of the neutrophil primary granules of newborns, the inability to assess the bpi and defensin peptide arsenal of neutrophil primary granules, and the inability to explain the decreased phagocytic and bactericidal activity of the neutrophil primary
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
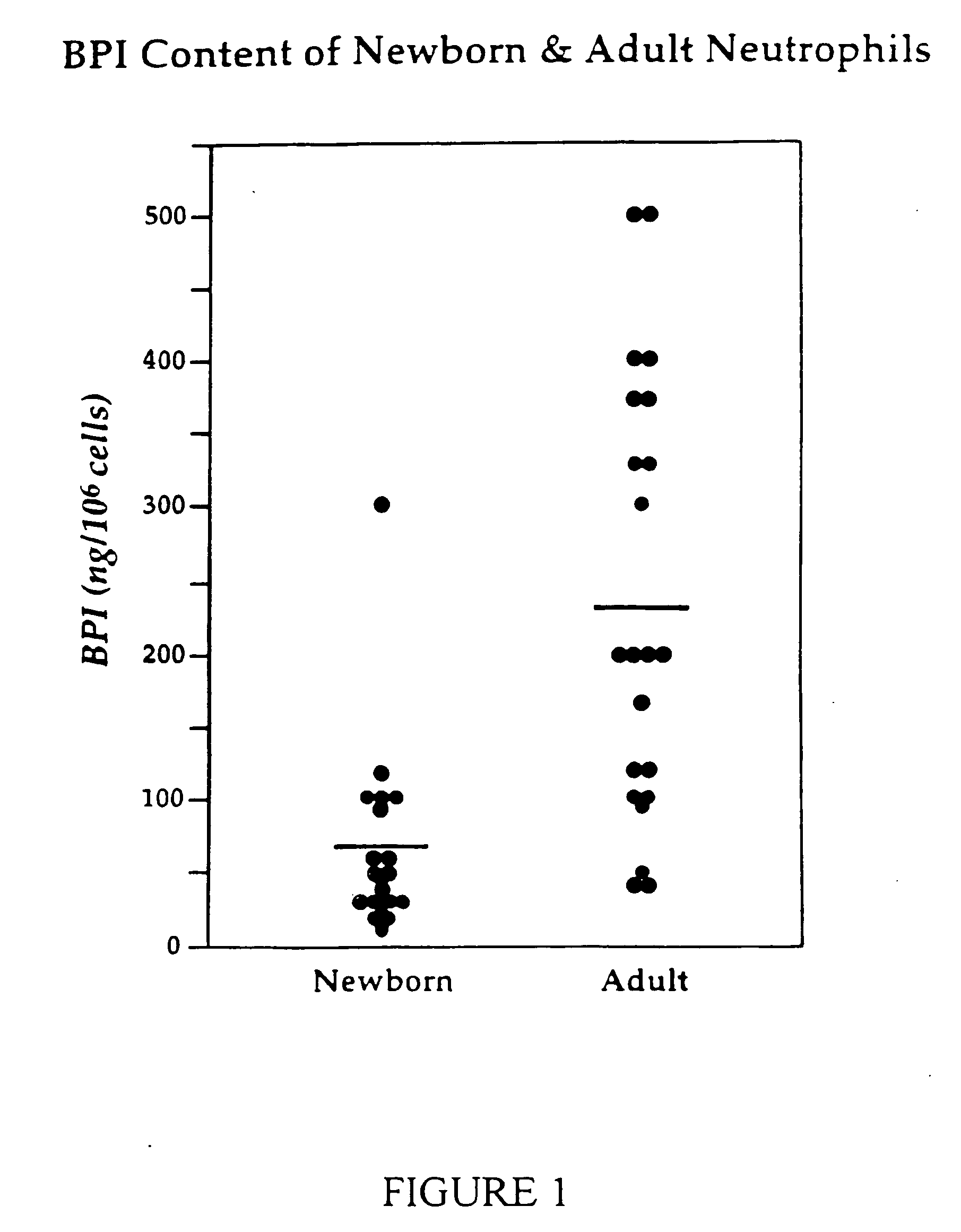
Comparison of BPI Content of Neonatal and Adult Neutrophils
[0041] In order to compare the BPI content of neonatal and adult neutrophils, cell-associated BPI was measured by Western blot analysis of neutrophil detergent extracts. The neutrophil content of BPI was then estimated by visual comparison to two-fold dilutions of purified BPI, allowing quantitation of sample values.
[0042] Neonatal neutrophils were obtained from cord blood samples, which were collected immediately after cesarean section or vaginal delivery. Cord blood was collected into sterile tubes anticoagulated with sodium heparin (Becton Dickinson) and placed on ice. All samples were labeled numerically and the results kept anonymous. Adult neutrophils were obtained from peripheral blood from healthy adult volunteers.
[0043] Neutrophils were isolated from whole blood as described in Le-v et al., J. Immunol 154: 5403-10 (1995). Anticoagulated blood was promptly (within 30-60 minutes) processed by dextran sedimentation ...
example 2
Comparison of Extracellular Levels of BPI in Neonatal and Adult Plasma
[0048] To determine whether the relatively low BPI content of newborn neutrophils was related to degranulation, possibly secondary to perinatal stress, the levels of extracellular BPI in newborn plasma samples and adult plasma samples were compared. Newborn and adult plasma samples were collected within 30-60 minutes of drawing cord or peripheral venous blood, respectively. Samples were stored in cryogenic microtubes (Sarstedt) at −70° C. prior to batch analysis.
[0049] BPI content of plasma was determined employing a biotinylated anti-BPI antibody in a sandwich ELISA format as described in White et al. (1994), supra. This ELISA system yielded a linear range from 0.1 to 6 ng BPI / ml and showed negligible cross reactivity with the homologous lipopolysaccharide-binding protein (LBP).
[0050] The average cord plasma BPI content was 16+ / −3 ng / ml (n=13), which is higher than that previously reported for plasma samples c...
example 3
Comparison of MPO and Defensin Levels in Neonatal and Adult Neutrophils
[0051] To assess whether other primary (azurophil) granule constituents were also relatively decreased in newborn neutrophils, the content of myeloperoxidase (MPO) and of defensin peptides in neonatal and adult neutrophils was measured as follows.
[0052] Levels of myeloperoxidase (MPO) were detected by Western blotting using 0.1% (v / v) rabbit anti-MPO serum [described in Nauseef et al., J Clin Invest 71: 1297-1307 (1983)] followed by 0.1% (v / v) I125 protein G. As control for MPO blots, a two-fold dose curve of adult azurophil granule fraction (prepared as described in Borregaard et al., J Cell Biol 97: 52-61 (1983) was solubilized in 4×SDS-PAGE buffer and analyzed as well. For purposes of quantitation, MPO content in neutrophil samples was expressed in “antigenic units” defined in relation to an adult azurophil granule extract standard: one antigenic unit was set equal to the band intensity of an azurophil granu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com