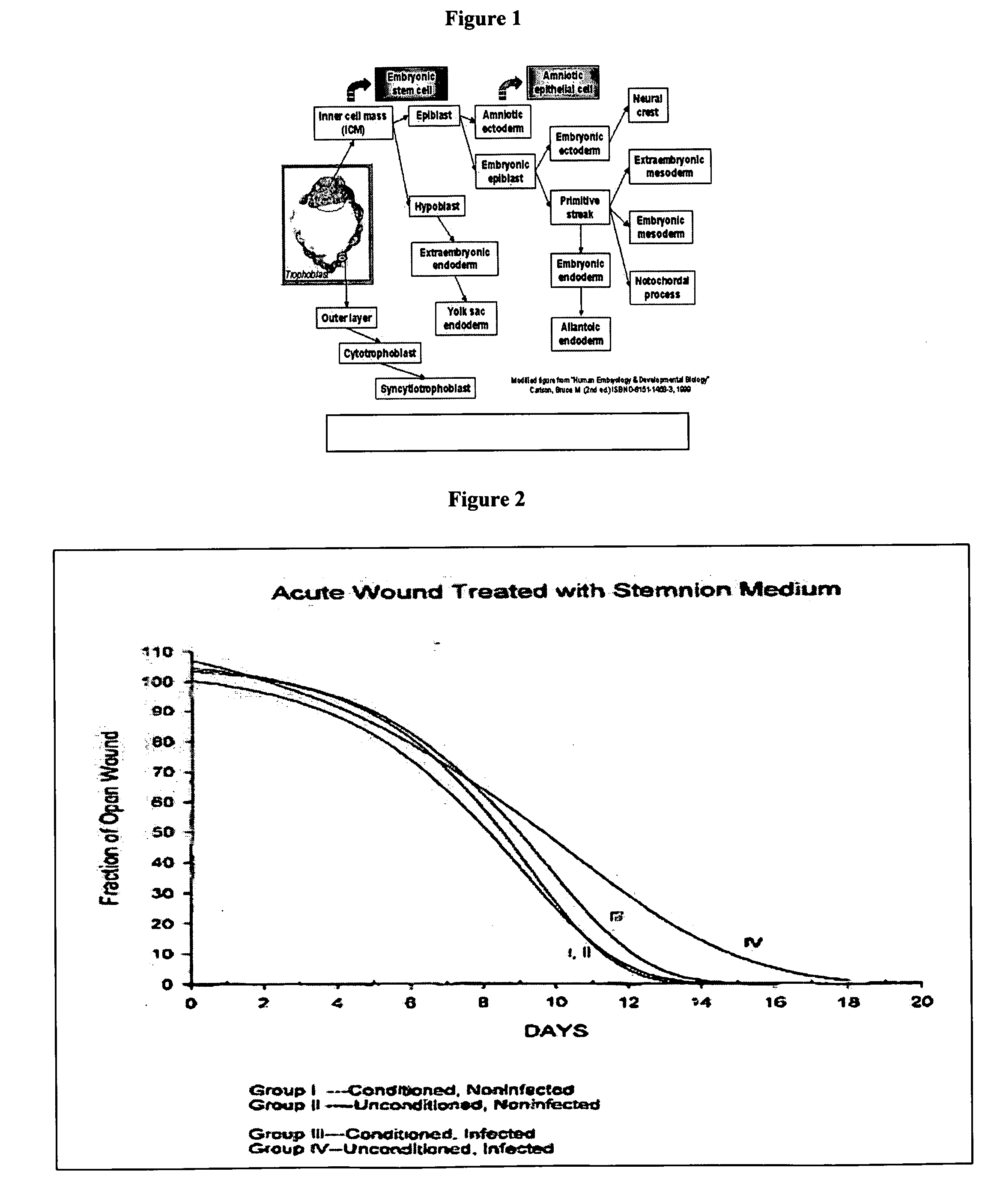
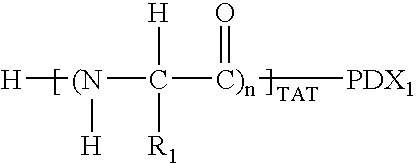Amnion-derived cell compositions, methods of making and uses thereof
a technology of amnion-derived cells and compositions, applied in the field of amnion-derived cell compositions, can solve the problems of incisional hernia, failure to mechanically fix, and failure to successfully heal acute wounds,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Amnion-Derived Cell Compositions
[0271] Recovery of amnion-derived cells—Amnion-derived cells were dissociated from starting amniotic membrane using the dissociation agents PXXIII, and trypsin. The average weight range of an amnion was 18-27 g. The number of cells recovered per g of amnion was about 10-15×106 for dissociation with PXXIII and 5-8×106 for dissociation with trypsin.
[0272] Culture conditions—The primary amnion-derived cells were cultured for 5 passages in the following media: Stemline II+10% FBS, Stemline II+10% plasbumin (pb), Ultraculture+10% plasbumin (pb), and DMEM+10% FBS. Each culture condition was tested using 15 million cells / g amnion, 10 million cells / g amnion, and 5 million cells / g amnion, depending on the enzyme used for recovery of the primary cells. For instance, using PXXIII, 15 million cells / g amnion were obtained, while using trypsin, 10 million cells / g amnion were obtained, while other enzymes resulted in even lesser recovery (5 million ...
example 2
Scale-Up of Amnion-Derived Cells on Microcarrier Beads in Spinner Flasks
[0277] Methods—One of the most common and oldest techniques for maintaining cells in suspension culture is by the use of spinner flasks. The cells can be either attached to microcarrier beads (adherent) or growing completely without any surface attachment (non-adherent). In either case, these flasks consist of a sterile vessel that contains a magnetic stirring mechanism that permits continuous stirring of the medium and cells under sterile conditions. This continuous stirring facilitates the diffusion of nutrients, promotes oxygenation of the medium, and eliminates concentration gradients. The vessels are stirred in a temperature-controlled, CO2 incubator.
[0278] Amnion-derived cells are an epithelial cell type that are anchorage-dependent which may interfere with or prevent their adaptation to a pure suspension system. Although amnion-derived cells may survive in suspension culture, the proliferation of these ...
example 3
Scale-Up of Amnion-Derived Cell Compositions in Suspension
[0281] Amnion-derived cells were cultured in ultra-low adherence tissue culture 6-well plates (Corning) in various mammalian cell culture media. These culture media were selected on the basis of their ability to promote proliferation of other mammalian cell types in suspension culture (i.e. 293S, Ultraculture, Opti-MEM). Additives to the culture medium in these experiments include a proprietary source of protein, and EGF (10-20 ng / ml) which preliminary experiments show is required for proliferation of amnion-derived cells. Amnion-derived cells were plated at a density of 1.3×106 cells / well, and the cultures were maintained at 37° C., in 5% CO2 in air. Culture medium was replaced every two days and cell number was assessed weekly. Preliminary experiments showed that amnion-derived cells sometimes form small floating clusters in suspension culture conditions. These clusters must be dispersed to ensure accurate cell counts and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com