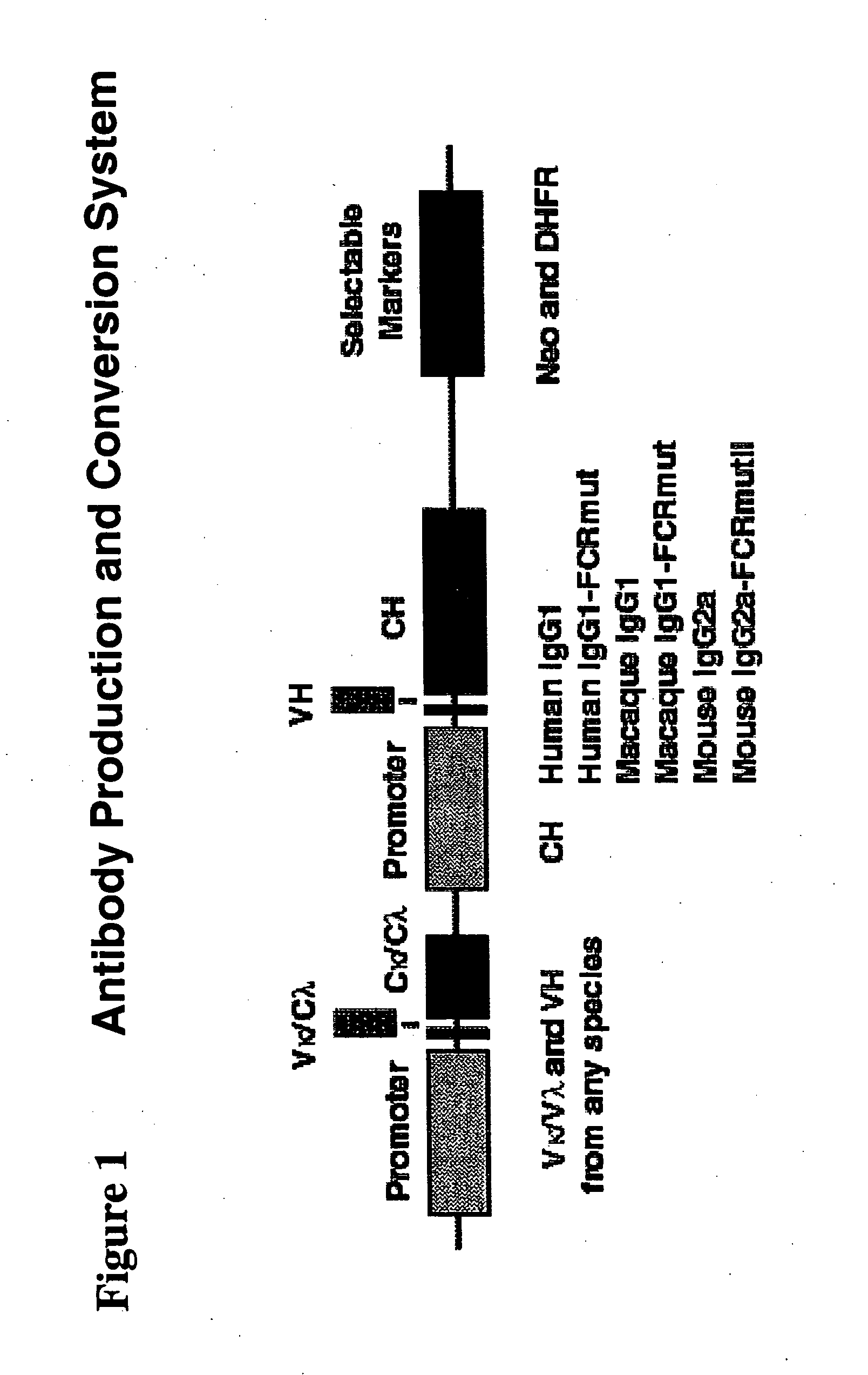
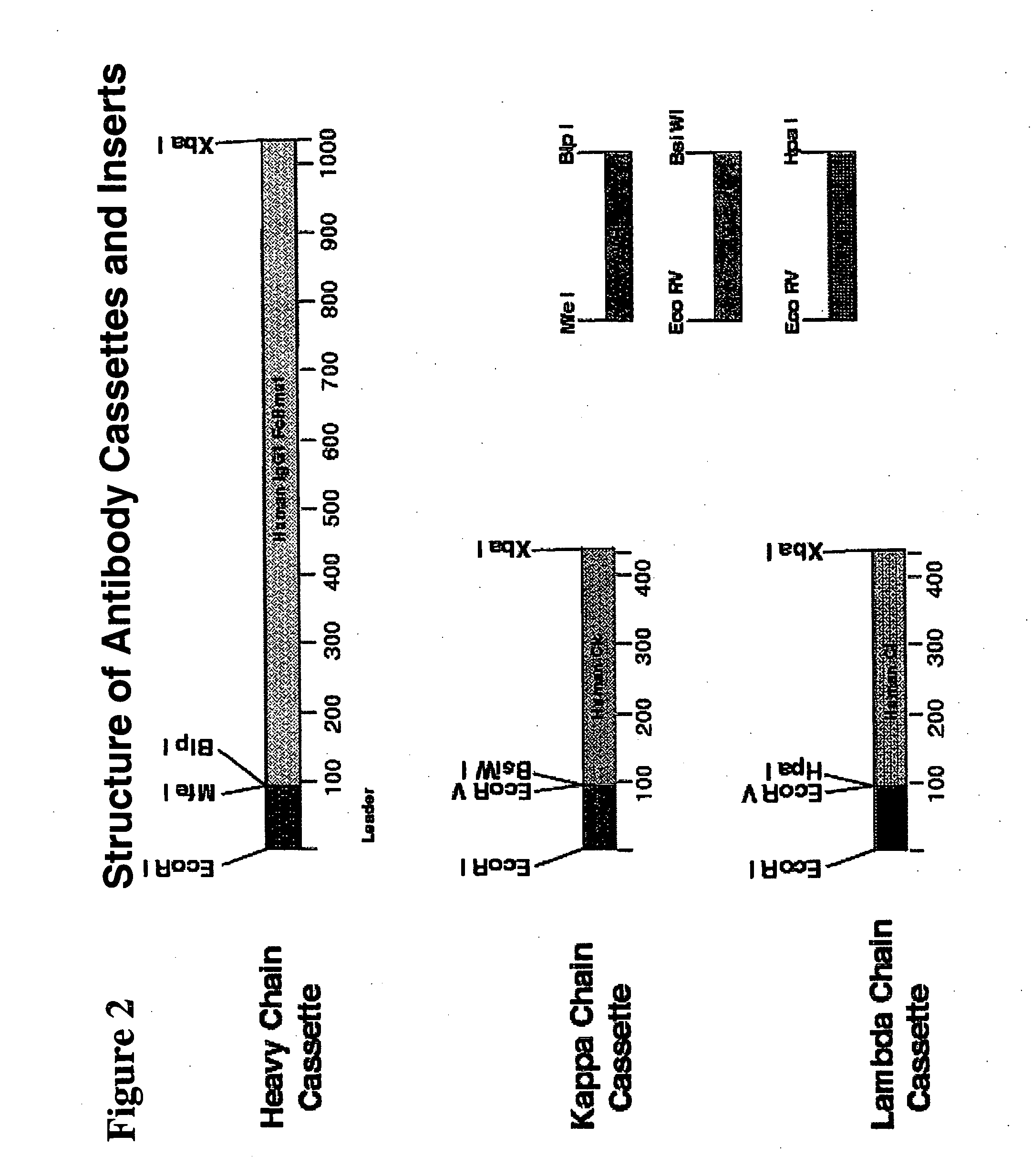
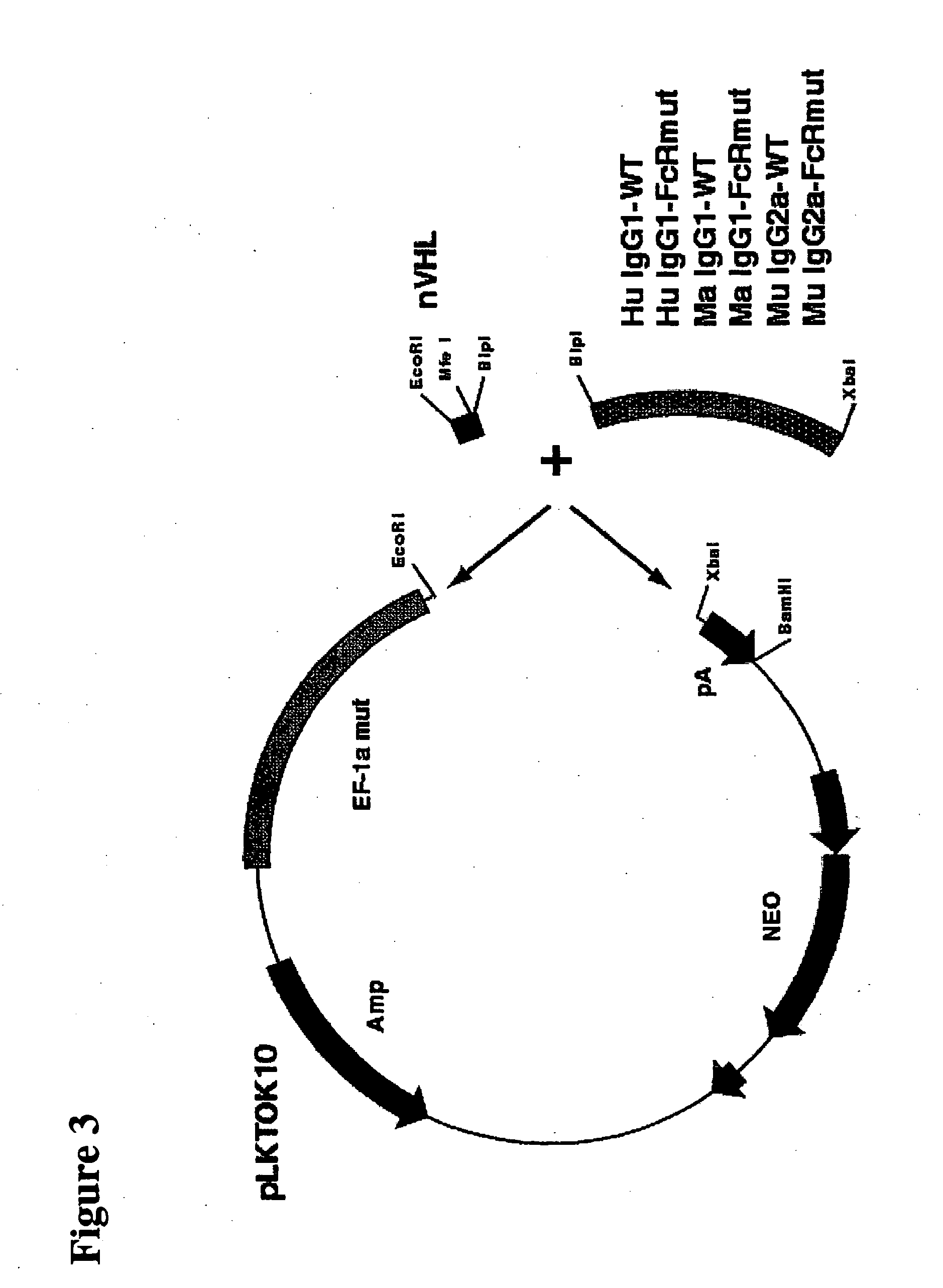
Immunoglobulin DNA cassette molecules, monobody constructs, methods of production, and methods of use therefor
a technology of immunoglobulin and cassette molecules, which is applied in the field of immunoglobulin dna cassette molecules, can solve the problems of time-consuming processes, inability to generate therapeutically useful antibodies, and inability to generate effective antigens in animals/humans, and achieves the effect of simple generation of desired immunoglobulin molecules and rapidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Creation of Leader Sequences
[0164] The VH heavy chain leader (nVHL) was created by reverse translation of human and mouse VH signal sequence available in the public database to determine those with the potential to have useful restriction enzyme cloning sites added within the signal sequence. The sequence chosen for the VH leader comes from the mouse gene U60820 which has been described previously (See Mus musculus anti-digoxin immunoglobulin heavy chain variable region precursor mRNA, partial cds. Genbank Submission (13-JUN.-1996) Mironova, R. S., et al.; Gene activity regulation, Inst. Molecular Biology, Bulgarian Academy of Sciences, Acad G. Bonchev bl 21, Sofia 1113, Bulgaria.) [0165] U60820 (SEQ ID NO: 101): MAVLGLLFCLVTLPNCVLS
[0166] DNA sequences were designed that would encode both the original protein sequence and the desired unique restriction cloning site, MfeI, flanked by an EcoRI restriction enzyme site and a Kozak sequence on the 5′ end. At its 3′ end a stuffer sequen...
example 2
Creation of Human Cassettes
[0174] The human heavy chain constant region, human IgG1-WT, was constructed by a standard PCR reaction of human splenic cDNA (Invitrogen) with the primers pCHhum1 and pCHhum2 (Table 2). The primer pCHhum1 contains the cloning site BlpI within its 5′ sequence and the primer pCHhum2 contains a stop codon followed by the cloning site XbaI. Utilization of these primers resulted in generation of the human constant region sequences shown in SEQ ID NO:7 & 8.
[0175] The human IgG1-WT cassette, named PLKTOK55, was then created by cloning the nVHL fragment together with the IgG1 fragment into the expression vector pLKTOK10 which contains an EF-1a promoter and a BGH polyadenylation region flanking the cloning site (FIG. 3). This method was used to create all the described heavy chain cassettes.
[0176] The entire region of promoter through polyadenylation region is flanked with BglII and BamHI restriction enzyme sites. This allows the entire cassette to be transferr...
example 3
Creation of Macaque Cassettes
[0185] Published sequences for the cynomologous and rhesus macaques IgG1 cDNA's were different at 3 amino acids so cDNA for IgG1 isolated from PBLs from both monkey species (See, e.g., Calvas P, et al. Characterization of the three immunoglobulin G subclasses of macaques. 1999 Scand J Immunol 49(6):595-610; and Lewis A P, et al. Cloning and sequence analysis of kappa and gamma cynomolgus monkey immunoglobulin cDNAs. 1993. Dev Comp Immunol 17(6):549-60). Sequencing analysis determined that the reported differences did not truly exist so a single macaque IgG1 cassette could be created for use in both monkey species.
[0186] The macaque heavy chain constant region, macaque IgG1-WT, was constructed by a standard PCR reaction of rhesus PBL cDNA with the primers pIgGrh1 and pIgGrh4 (Table 2). The primer pIgGrh1 contains the cloning site BlpI within its 5′ sequence and the primer pIgGrh4 contains a stop codon followed by the cloning site XbaI. Resultant sequenc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| molar ratios | aaaaa | aaaaa |
| nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com