Atopic dermatitis inducer
a technology of inducer and atopic dermatitis, applied in the field of inducer, can solve the problems of not being able to provide essential treatment and not paying attention to the substances produced in the body, and achieve the effect of reducing symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
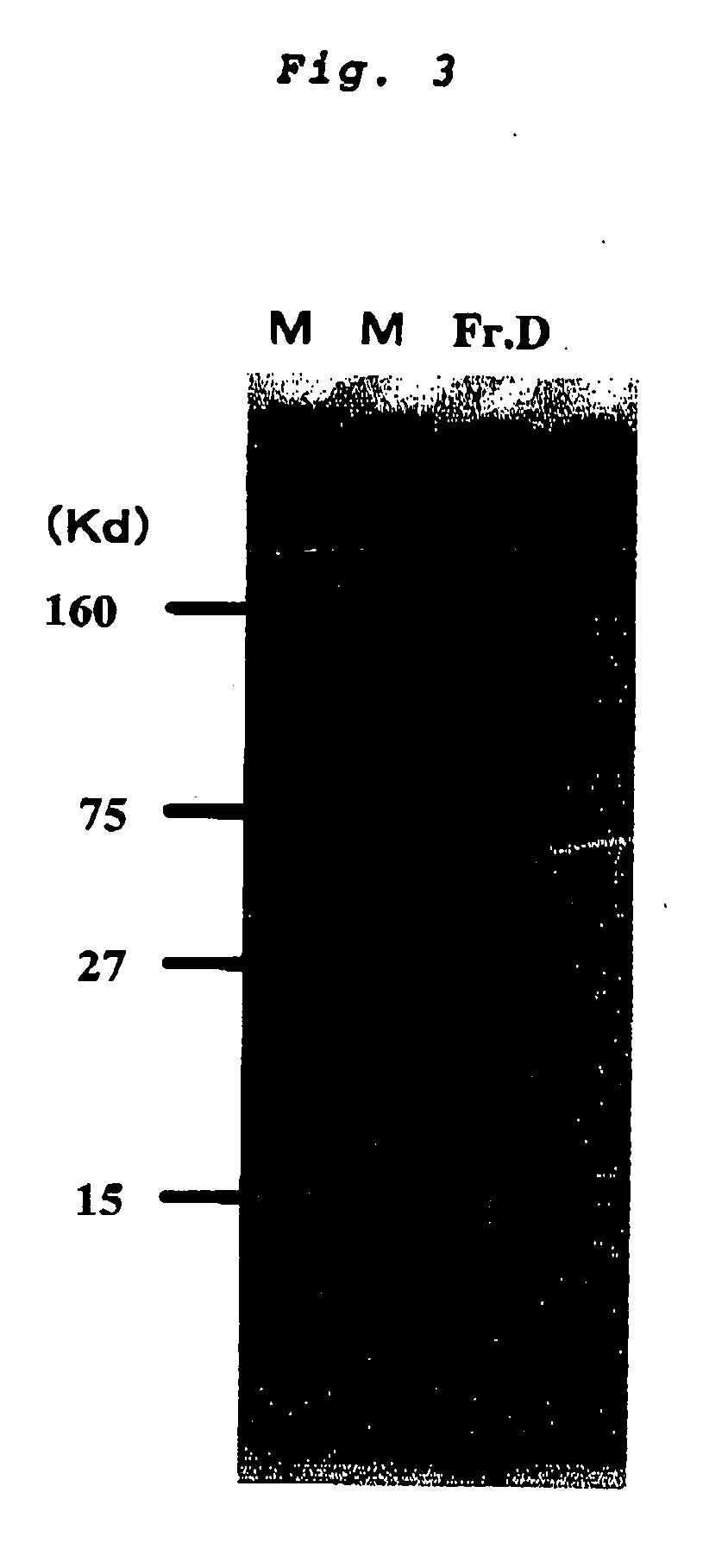
[0077] Purification of Fraction Having Histamine-Releasing Activity (Fr.D)
1-1. Step of Filtering Human Sweat, Removing Insoluble Matters and Collecting the Filtrate
[0078] 500 ml of human sweat was filtered (0.22 μm), and the precipitate was removed. A filtrate with a total protein amount of 1.07 mg roughly estimated from the optical density (OD, 280 nm) was obtained,
1-2. Step of Mixing the Filtrate with a ConA-Affinity Carrier and Collecting the Supernatant
[0079] To the sweat subjected to filtering, 24 ml of a ConA-affinity carrier (ConA-Sepharose, Amersham Pharmacia; glycoprotein adsorption capacity: 45 mg / ml) was added, and stirring was carried out overnight at 4° C., and then the supernatant (a fraction not adsorbed to ConA-Sepharose) was collected.
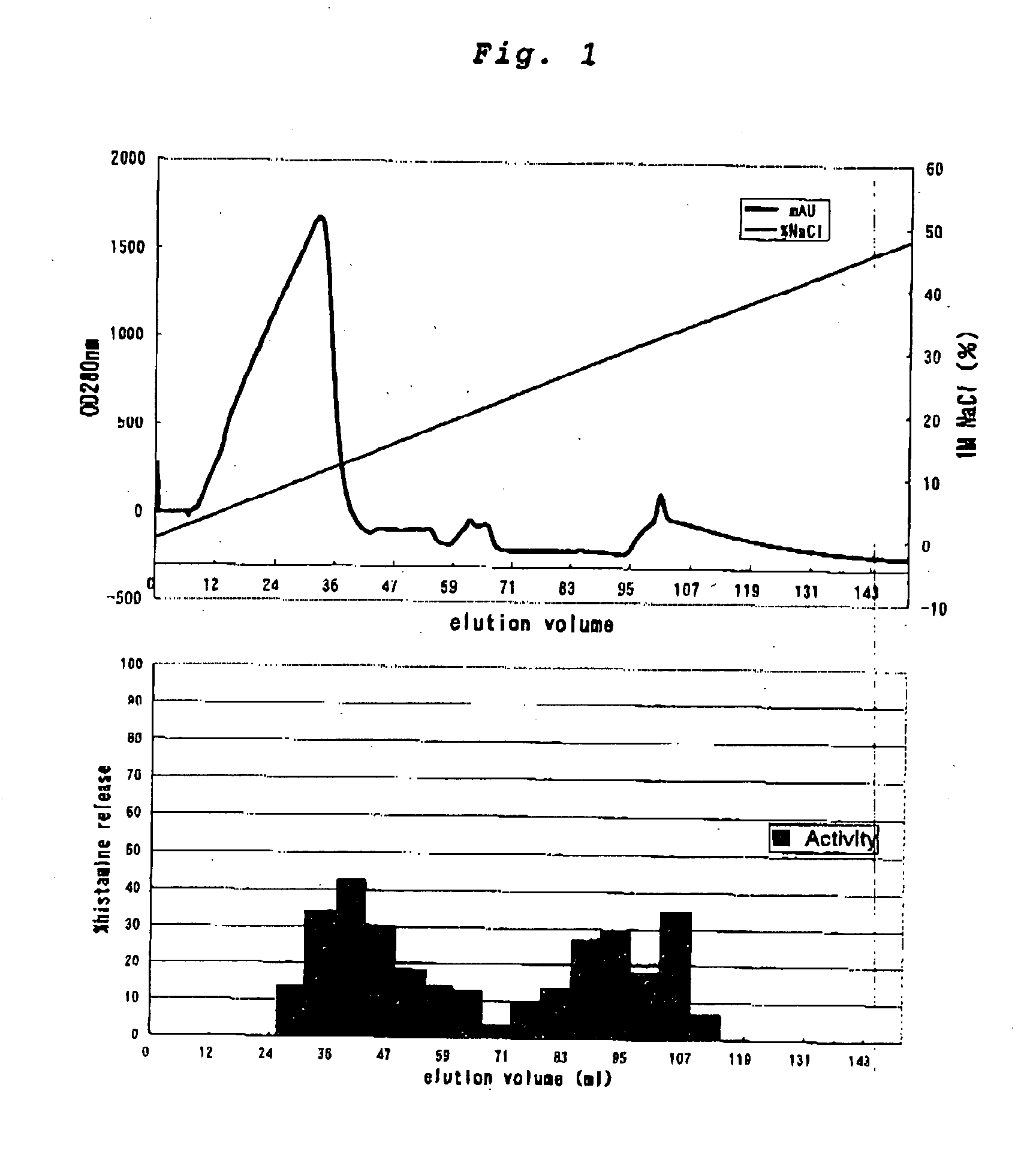
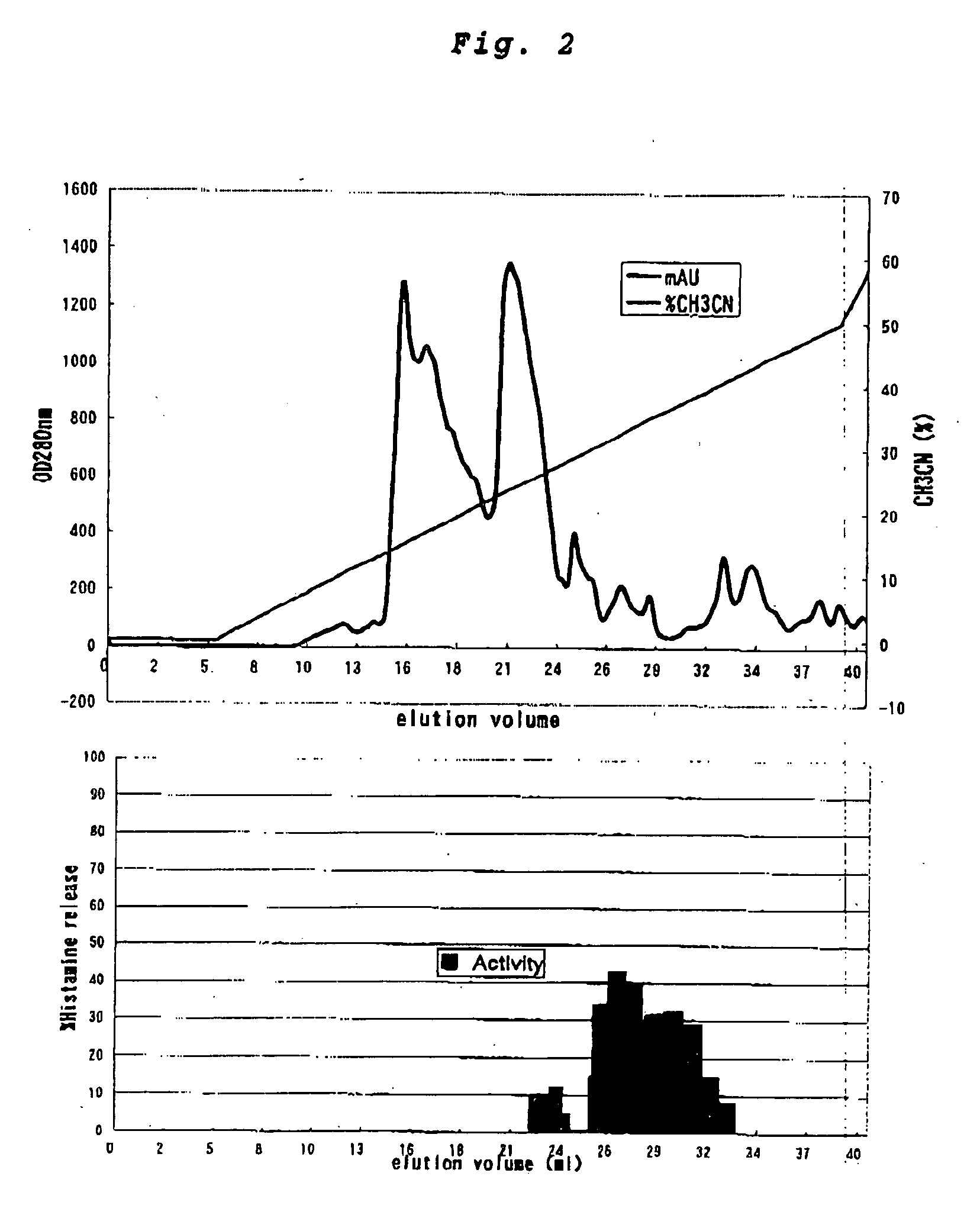
1-3. Step of Separating the Supernatant with an Anionic Exchange Column and Collecting a Fraction Having a Histamine-Releasing Activity
[0080] Onto an anionic exchange column (MonoQ HR 10 / 10; Pharmacia Biotech), which had been ...
example 2
Effect of Serum of Patient with Atopic Dermatitis on Histamine-Releasing Activity of Fr.D
[0083] Fr.D was collected, lyophilized, redissolved in PBS at a concentration of 1 mg / ml and used as an Fr.D standard. When 10 μl of a 50-fold dilution of the Fr.D standard was added to 40 μl of the serum of a patient with atopic dermatitis and preincubation was carried out at 37° C. for 30 minutes, the histamine-releasing activity was completely lost. On the contrary, when the serum of a normal subject was added, the histamine-releasing activity was not lost (FIG. 5). “antiIgE” and “Fr.D (×500)” are a positive control, and “serum (AD)” (serum of a patient) and “serum (N)” (serum of a normal subject) are a negative control.
example 3
Enzyme Treatment
3-1. DNase Treatment and Lipase Treatment
[0084] To 5 μl of the Fr.D standard, DNase (Ambion) was added at a final concentration of 0.2 U / μl, or a pancreatic lipase (SIGMA) was added at a final concentration of 30 mU / μl, and incubation was carried out at 37° C. for 16 hours. In both cases, a decrease in the histamine-releasing activity was not observed.
3-2. Protease Treatment
[0085] To 5 μl of the Fr.D standard, 2 μg / μl of ProteinaseK (Ambion) or 2 μg / μl of Trypsin (SIGMA) was added to give a final concentration of 0.1 μg / μl, and incubation was carried out at 37° C. for 16 hours. In both cases, the histamine-releasing activity was completely lost.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com