Genetic suppression and replacement
a gene and gene technology, applied in the field of gene suppression and replacement, can solve the problems of difficult targeting of disease mutations, inability to develop specific gene therapies for each of them, and inability to differentiate strategies,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A: Human Rhodopsin
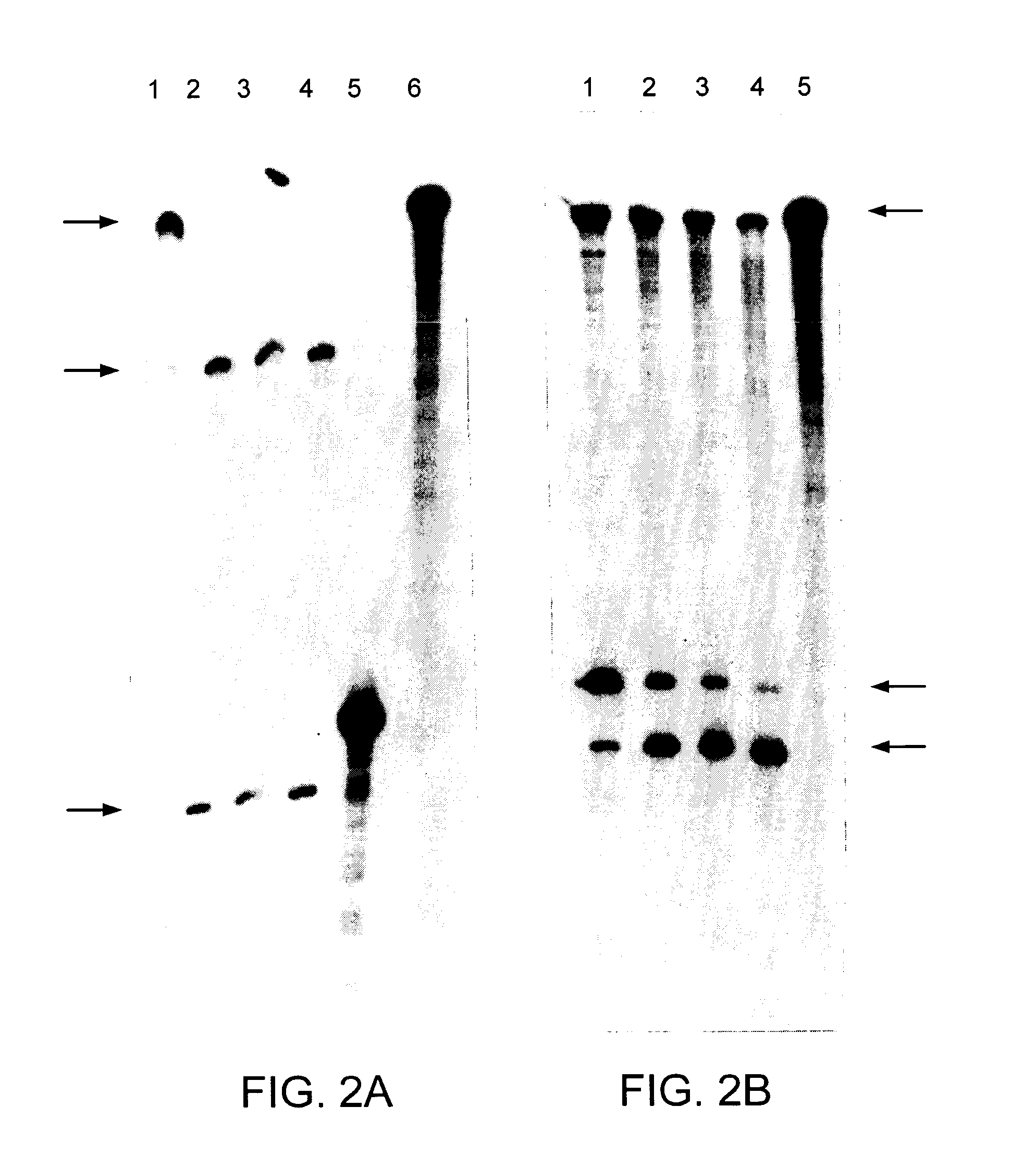
[0111] The unadapted human rhodopsin CDNA (SEQ ID NO:1) and the human rhodopsin CDNA with a single nucleotide substitution in the coding sequence (SEQ ID NO:2) were cut with BstEII and expressed in vitro. The single base change occurs at the third base position or wobble position of the codon (at position 477) (nucleotide 271 of SEQ ID NO:2) and therefore does not alter the amino acid coded by this triplet. The Rz10 clone was cut with XbaI and expressed in vitro. Resulting ribozyme and human rhodopsin RNAs were mixed with varying concentrations of MgCl2 to optimise cleavage of template RNA by Rz10. A profile of human rhodopsin RNA cleavage by Rz10 over time is given in FIG. 2A. The MgCl2 curve profile used to test if adapted human rhodopsin transcripts could be cleaved by Rz10 is given in FIG. 3B. Unadapted and adapted human rhodopsin cDNAs were cut with FspI and BstEII respectively, expressed and mixed together with Rz10 RNA to test for cleavage (FIG. 2B) over t...
example 2
Mouse Rhodopsin
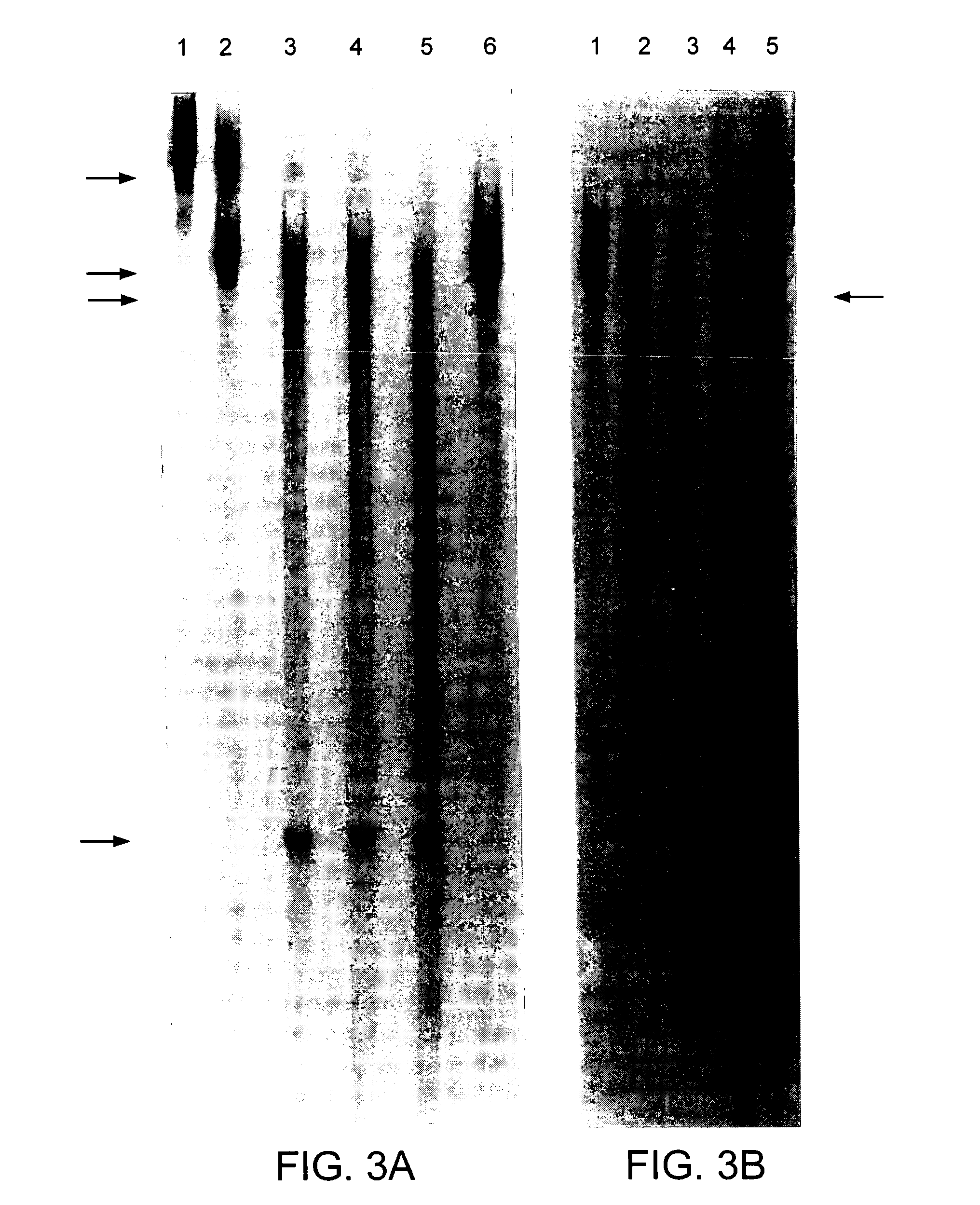
[0114] Rz33 was cut with XbaI and expressed in vitro. Similarly the mouse rhodopsin cDNA was cut with Eco47III and expressed in vitro. Resulting RNAs were mixed and incubated with varying concentrations of MgCl2. All expressed products and cleavage products were the correct size. FIG. 6A demonstrates specific cleavage of the mouse rhodopsin RNA over various MgCl2 concentrations incubated at 37° C. for 3 hours. Using a replacement gene with a sequence change around the Rz33 cleavage site (TTT-->TCT) (nucleotides 189-191 of SEQ ID NO:7) which is at a wobble position we demonstrated that transcripts from the replacement gene remain intact due to absence of the Rz33 target site (FIG. 6B). Hence Rz33 could be used to cleave mutant transcripts in a manner independent of the disease mutation itself (that is, using this site) while transcripts from the replacement gene which code for the correct protein would remain intact and therefore could supply the wild type protein.
example 3
Human Peripherin
[0115] The unadapted human peripherin cDNA and two human peripherin DNA fragments generated by PCR mutagenesis with a single nucleotide substitution in the coding sequence were cut with BglII and AvrII respectively and expressed in vitro. The single base changes in the adapted DNAs occur at third base positions or wobble positions of the codon (at position 257 and 359) (nucleotide 468 of SEQ ID NO:13 and nucleotide 332 of SEQ ID NO:10, respectively) and therefore do not alter the amino acid coded by these triplets. The Rz30 and Rz31 clones were cut with XbaI and expressed in vitro. Resulting ribozymes and unadapted human rhodopsin RNAs were mixed with varying concentrations of MgCl2 to optimise cleavage of template RNA by Rz30 and Rz31 . Profiles of human peripherin RNA cleavage by Rz30 over various MgCl2 concentrations and over time are given in FIG. 7. Similarly profiles of human peripherin RNA cleavage by Rz31 over various MgCl2 concentrations and over time are g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular defects | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com