Method for purifying protein and glucose dehydrogenase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
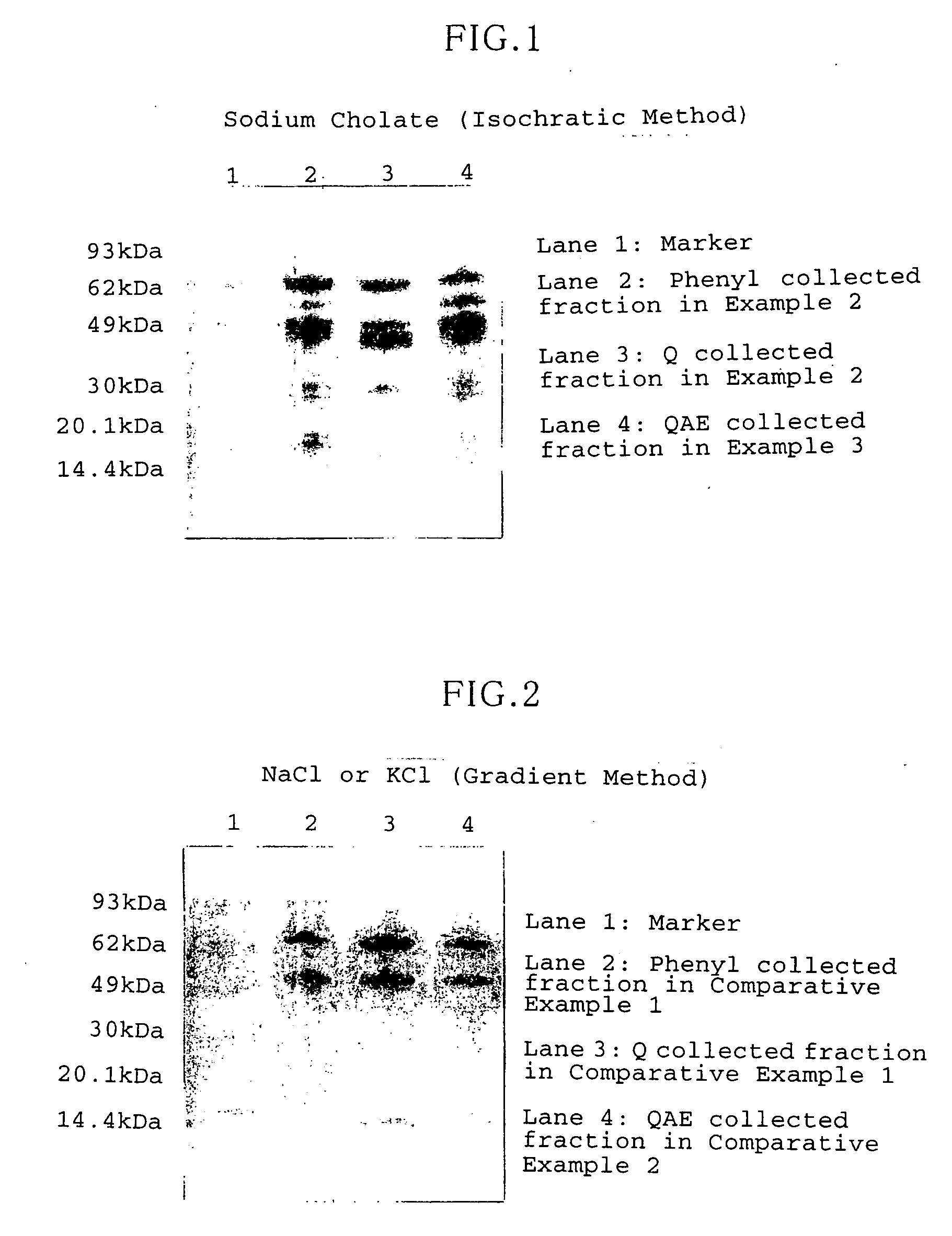
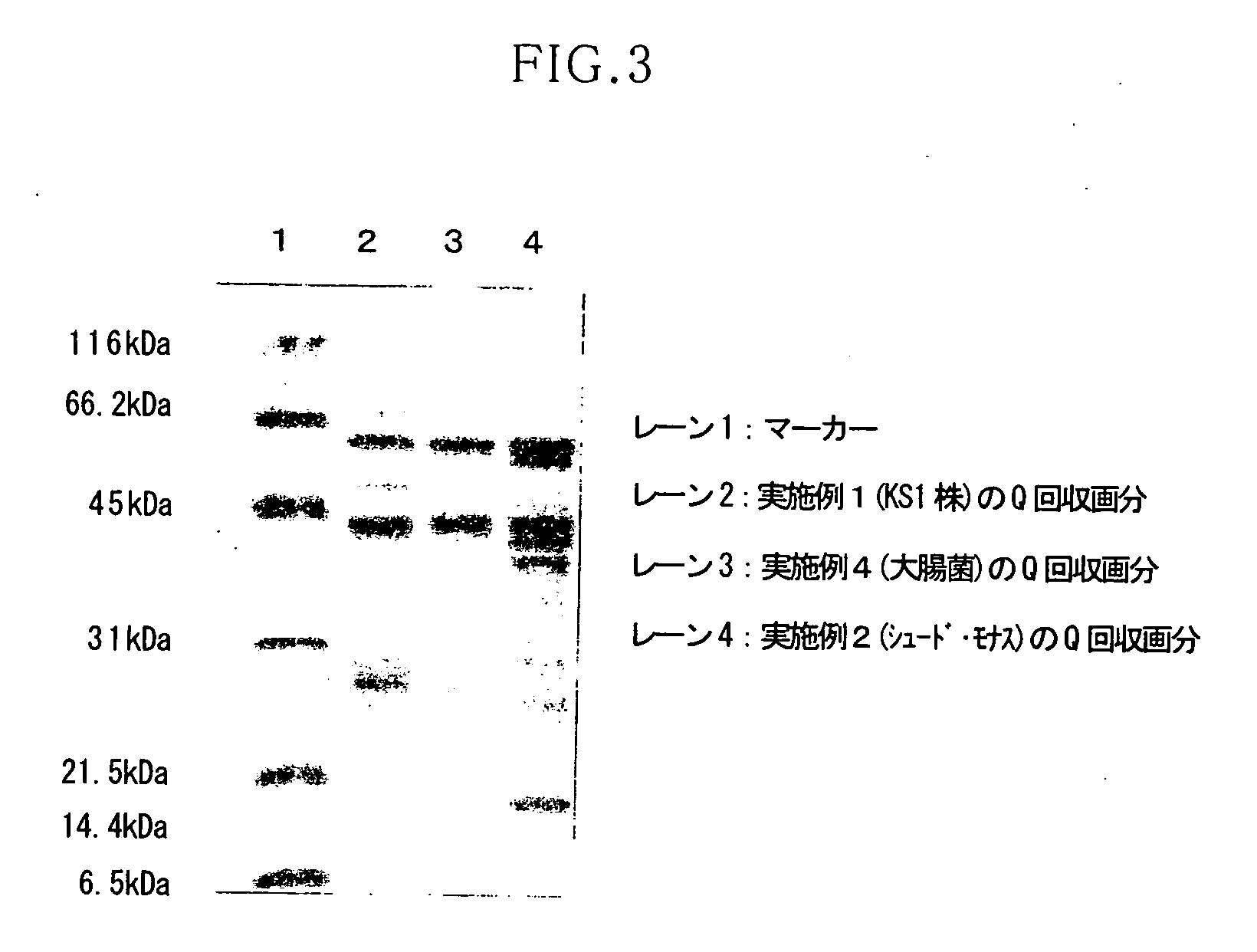
[0064] In this Example, the enzyme solution (solubilized GDH fraction) which was obtained from KS1 strain according to the above-described technique was purified by using a combination of hydrophobic chromatography and anion exchange chromatography.
[0065] The hydrophobic chromatography was performed by using an Octyl sepharose 4 Fast Flow column (44 mm ID×20 cm Amersham Bioscience KK) which had been equilibrated with 60 mM phosphate buffer solution (pH6). The column was supplied with solubilized GDH fraction (enzyme solution) which was prepared by adding 1 M phosphate buffer solution (pH 6) to achieve the final concentration of 60 mM. Next, 600 mL of 60 mM phosphate buffer solution (pH 6) and 900 mL of 20 mM phosphate buffer solution (pH 8) were passed, and then tightly adsorbed GDH was eluted by supplying eluent. The eluent was provided by 20 mM phosphate buffer solution (pH 8) which contained sodium cholate in dissolved form. The eluent was supplied at a rate of 15 mL / min so that...
example 2
[0070] In this Example, the crude enzyme solution obtained from the transformant of Pseudomonas putida was purified by using a combination of hydrophobic chromatography and anion exchange chromatography.
[0071] In the hydrophobic chromatography, first, a Phenyl cellulofine column (300 mm ID×10 cm, Chisso Corporation, Tokyo) which had been equilibrated with 10 mM phosphate buffer solution (pH 8) containing 0.1 M KCl was supplied with the crude enzyme solution, to let the packing agent hold GDH. Next, 7 L of 10 mM phosphate buffer solution (pH 8) containing 0.1 M KCl, and 21 L of 10 mM phosphate buffer solution (pH 8) were passed. Then, tightly adsorbed GDH was eluted by passing eluent. The eluent was provided by a 20 mM phosphate buffer solution (pH 8) containing sodium cholate in dissolved form. The eluent was supplied at a rate of 7 L / min so that sodium cholate concentration would vary linearly in the range of 0-1 wt %.
[0072] As a result, GDH was eluted when the sodium cholate con...
example 3
[0077] In this Example, the crude enzyme solution obtained from the transformant of Pseudomonas putida was purified by using a combination of hydrophobic chromatography and anion exchange chromatography.
[0078] The hydrophobic chromatography was performed in the same way as in Example 2. The collected solution was ultracondensed, to yield 70 mL of phenyl collected fraction, which had a specific activity of 300 U / mg and a total activity of 21 kU.
[0079] The anion exchange chromatography was performed by using a QAE-TOYOPEARL 550 column (44 mm ID×10 cm, Tohso Corporation, Tokyo) which had been equilibrated with 10 mM phosphate buffer solution (pH8). The column was supplied with the phenyl collected fraction, and then with eluent. The eluent was provided by 10 mM phosphate buffer solution (pH8) containing 1 wt % sodium cholate. The eluent was supplied at a rate of 5 mL / min and by a volume of 2000 mL.
[0080] As a result, GDH was eluted specifically when approximately 1600 mL of the elue...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com