Methods and compositions for ameliorating the undesirable effects of chemotherapy
a technology of undesirable effects and compositions, applied in the direction of drug compositions, dipeptide ingredients, medical preparations, etc., can solve the problems of affecting the health of patients, ototoxicity, seizures, etc., and achieve the effects of reducing the risk of recurrence, reducing and improving the effect of recurren
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
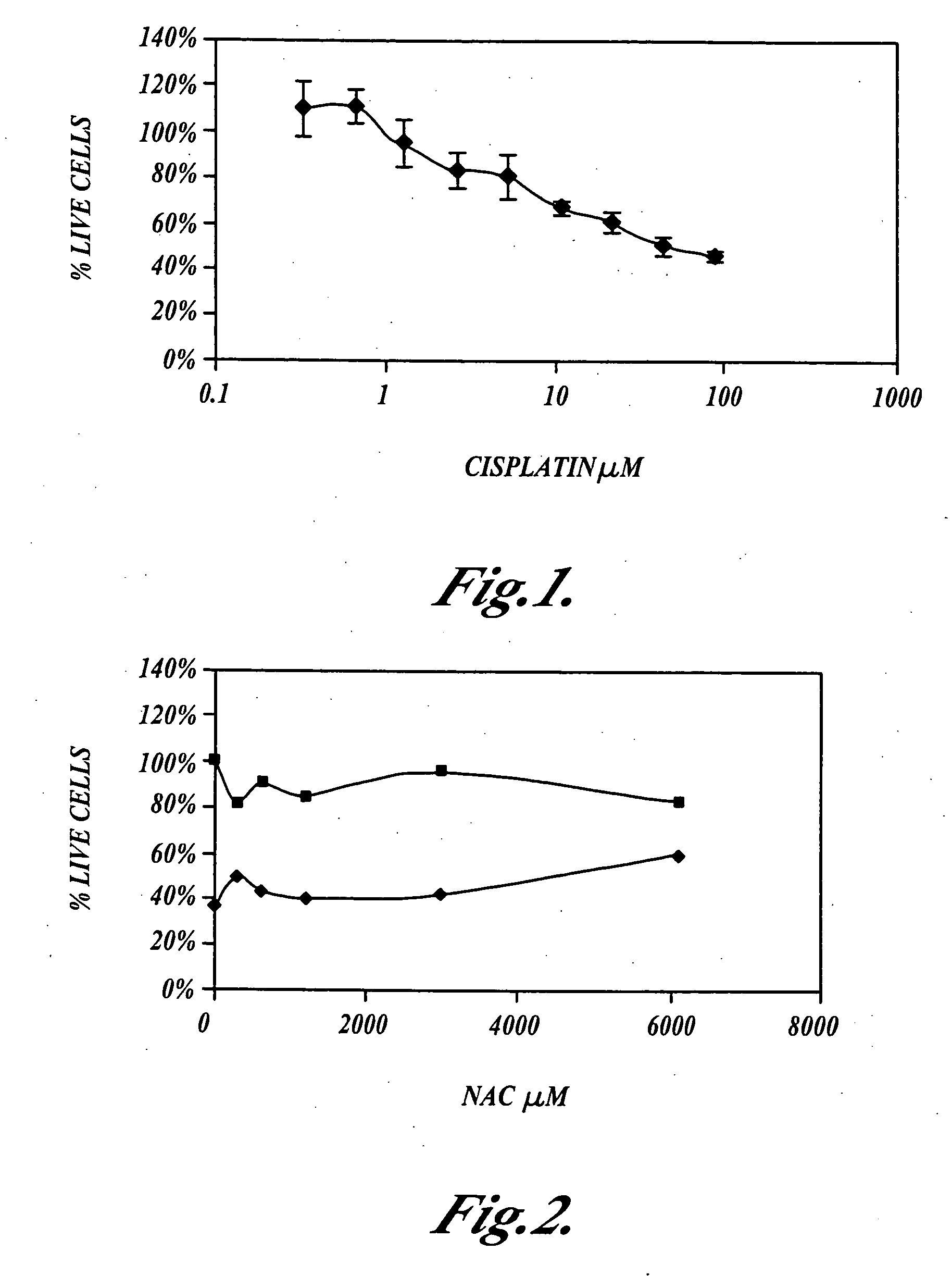
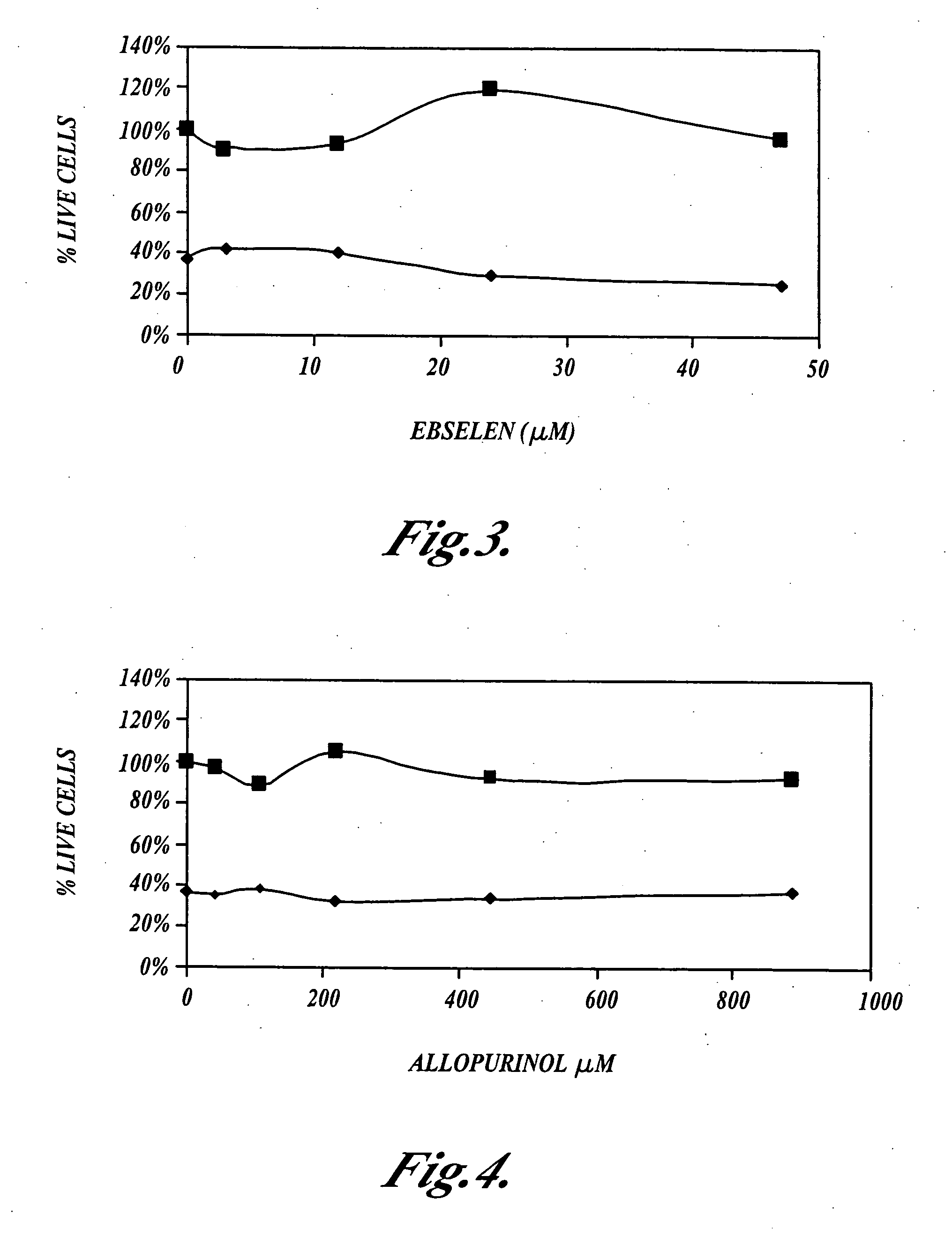
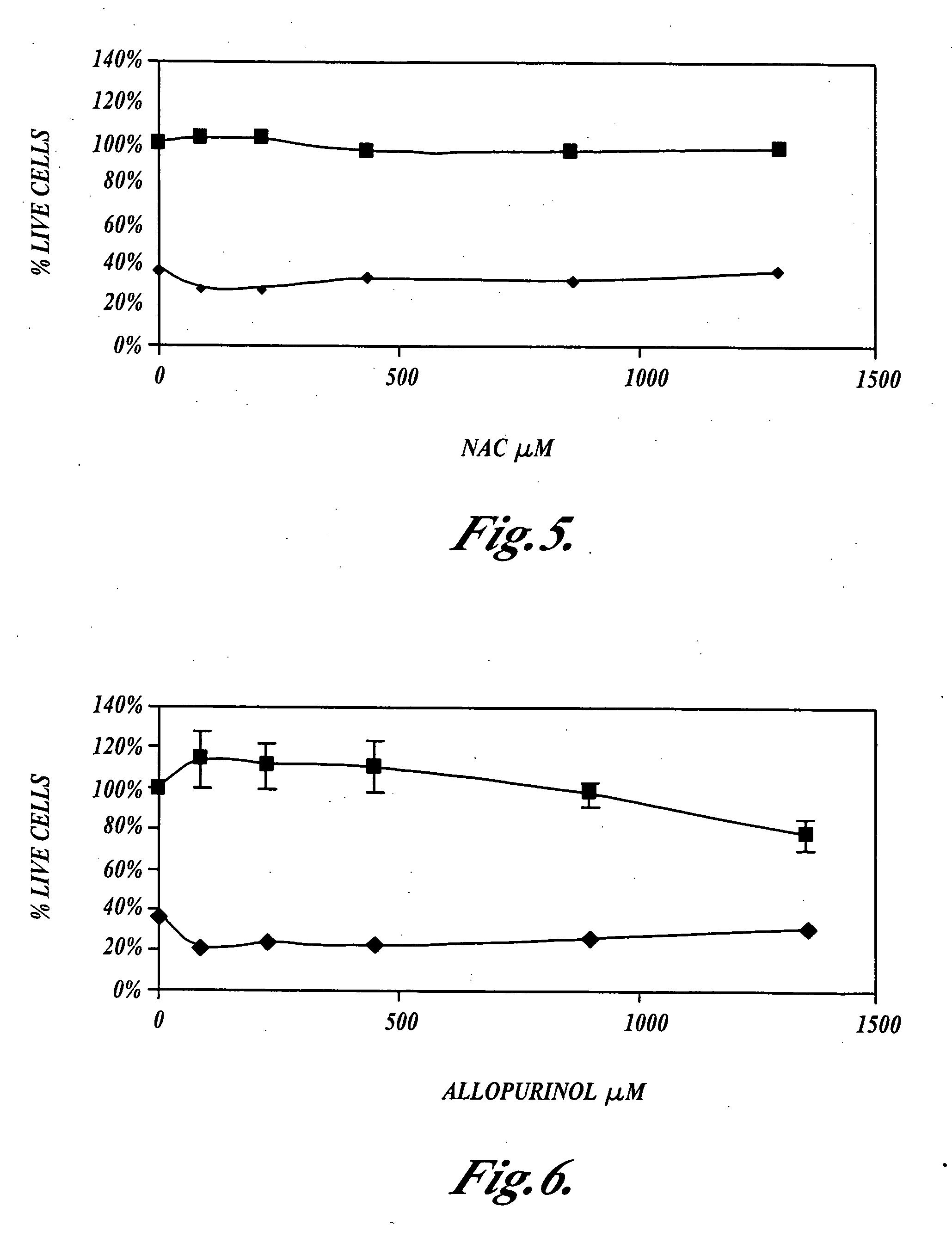
[0057] This example shows that N-acetylcysteine, Ebselen and allopurinol, alone, or in combination, do not inhibit the ability of cisplatin to kill cultured NuTu-19 ovarian cancer tumor cells as measured using the MTS cell viability assay.
[0058] NuTu-19 cells were plated at a density of 3,000 cells per well in 96 well culture dishes, and incubated at 37° C., in the presence of 5% carbon dioxide, for 24 hours. N-acetylcysteine, Ebselen or allopurinol were incubated for one hour, or for four hours, with the NuTu-19 cells, then cisplatin was added to the cultures which were further incubated at 37° C., in the presence of 5% carbon dioxide, for 24 hours. The NuTu-19 cells were then rinsed with media and incubated in the presence of cisplatin for an additional 24 hours.
[0059] The NuTu-19 cells were then rinsed twice with phosphate buffered saline (PBS), then MTS assays were performed to measure the number of living cells. MTS is an abbreviation for (3-(4,5-dimethylthiazol-2-yl)-5-(3-ca...
example 2
[0066] This Example shows that Ebselen protects inner ear hair cells from damage by cisplatin in vitro.
[0067] Three cochlea per treatment, obtained from P3-4 mouse pups, were cultured in 0.4 micrometer MilliCell-CM inserts with NeuroBasal A medium plus B27 supplement. After 24 hours in culture Ebselen was added to the medium, incubated for ten minutes, and then cisplatin was added to the medium at a final concentration of 43 μM. A first control treatment included 43 μM cisplatin. A second control treatment included 47 μM Ebselen without the addition of cisplatin. All cultures were incubated for 24 hours at 37° C. in 5% carbon dioxide.
[0068] The explants were then harvested, fixed, and stained with calbindin (which detects hair cells) and DAPI (4′,6-Diamindino-2-phenylindole; for detection of nuclear DNA). FIG. 7 shows the number of inner ear hair cells in mice cochlea that were cultured, in vitro, in the presence of 43 μM cisplatin (10), or 43 μM cisplatin plus 47 μM Ebselen (12),...
example 3
[0070] This Example shows that Ebselen, and the combination of Ebselen and allopurinol, protect rat inner ear hair cells from damage by cisplatin in vivo.
[0071] Auditory Evoked Brainstem Response (ABR) was used to assess hearing in rats before and after exposure to cisplatin and chemoprotectants. Ebselen or DMSO (control vehicle) were introduced intraperitoneally into rats one hour before intraperitoneal administration of cisplatin at a dosage of 16 mg / kg body weight. Seventy two hours after delivery of cisplatin, ABR data were collected, animals were sacrificed, cochleae were collected, dissected, stained with FITC-phalloidin (to detect F-Actin in hair cells), and DAPI (to detect nuclear DNA).
[0072]FIG. 8 shows the permanent threshold shift (PTS) in hearing, at 8 kHz, 16 kHz, 24 kHz and 32 kHz, of rats treated with cisplatin (at a dosage of 16 mg / kg body weight) in the presence of Ebselen (at a dosage of 16 mg / kg body weight) (22), or in the presence of saline and DMSO (control) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| chemoprotectant compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com