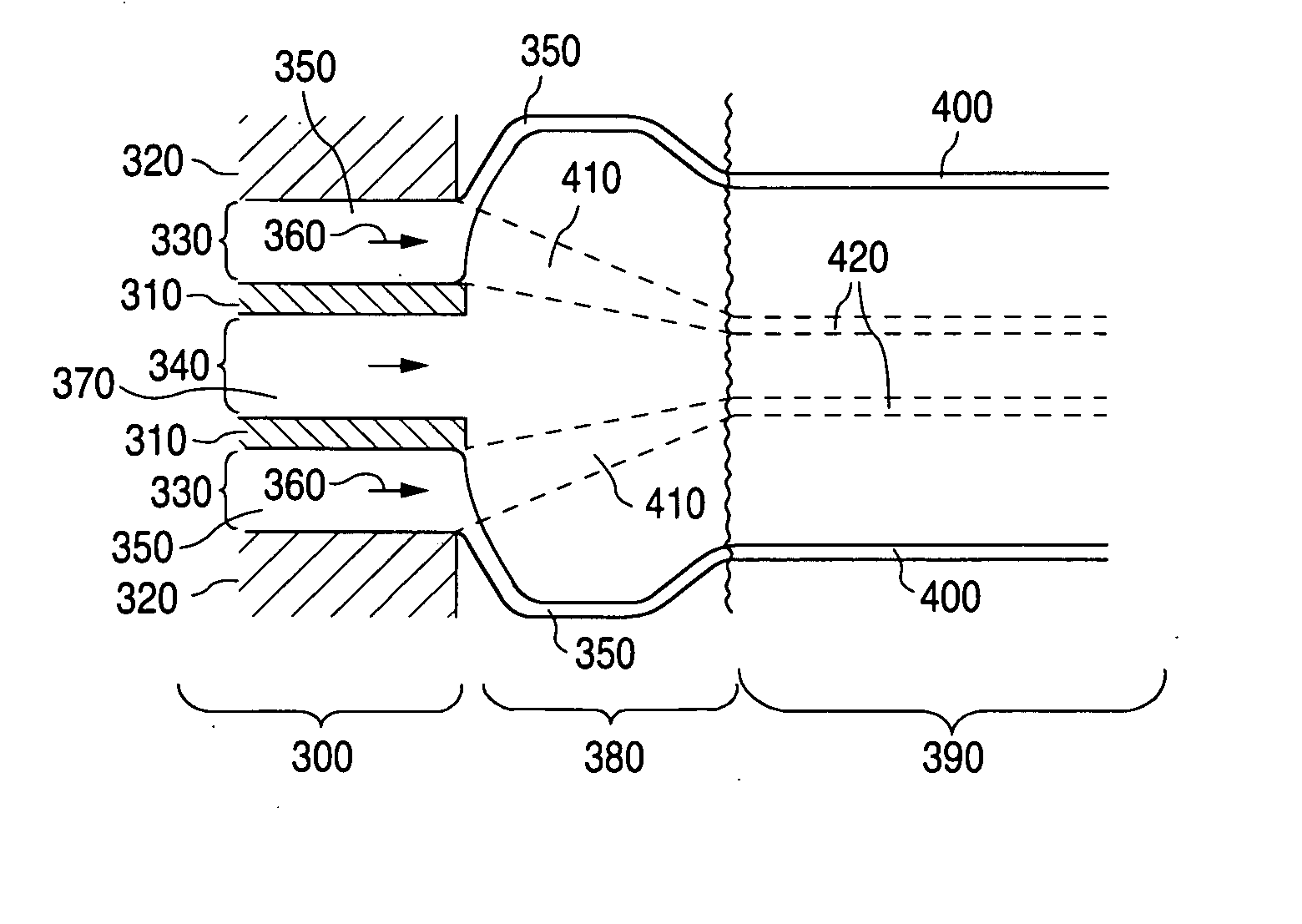
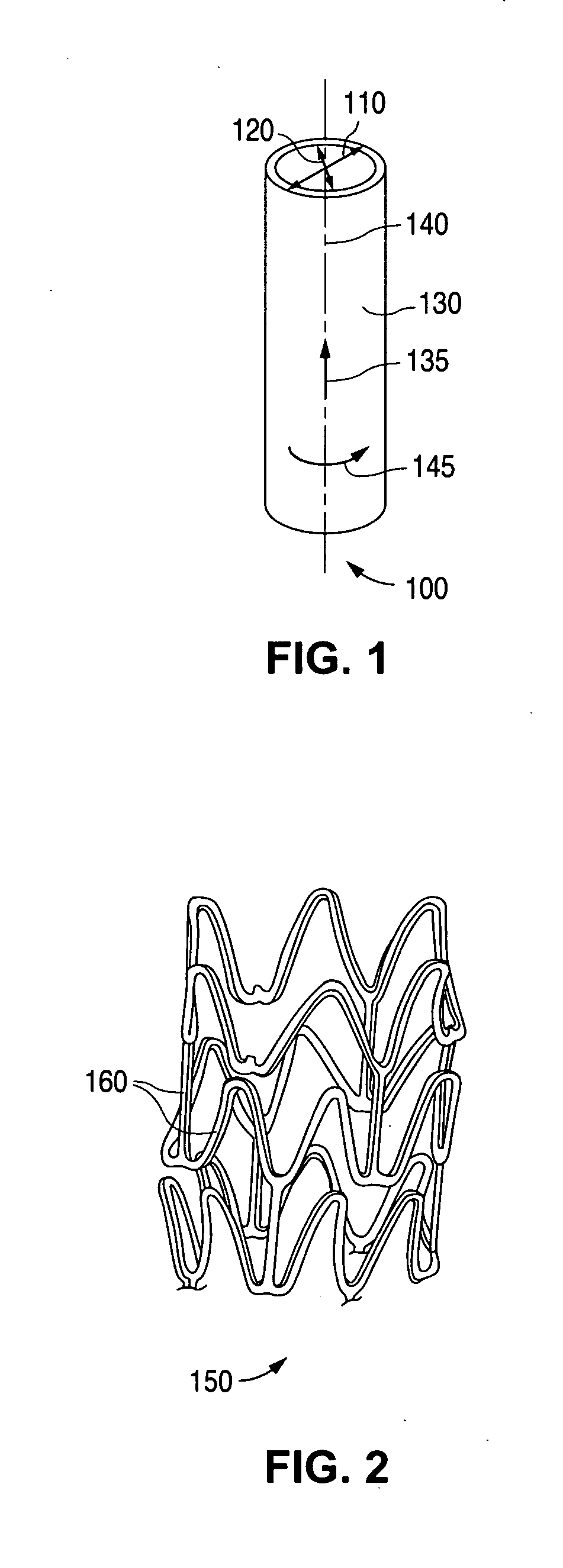
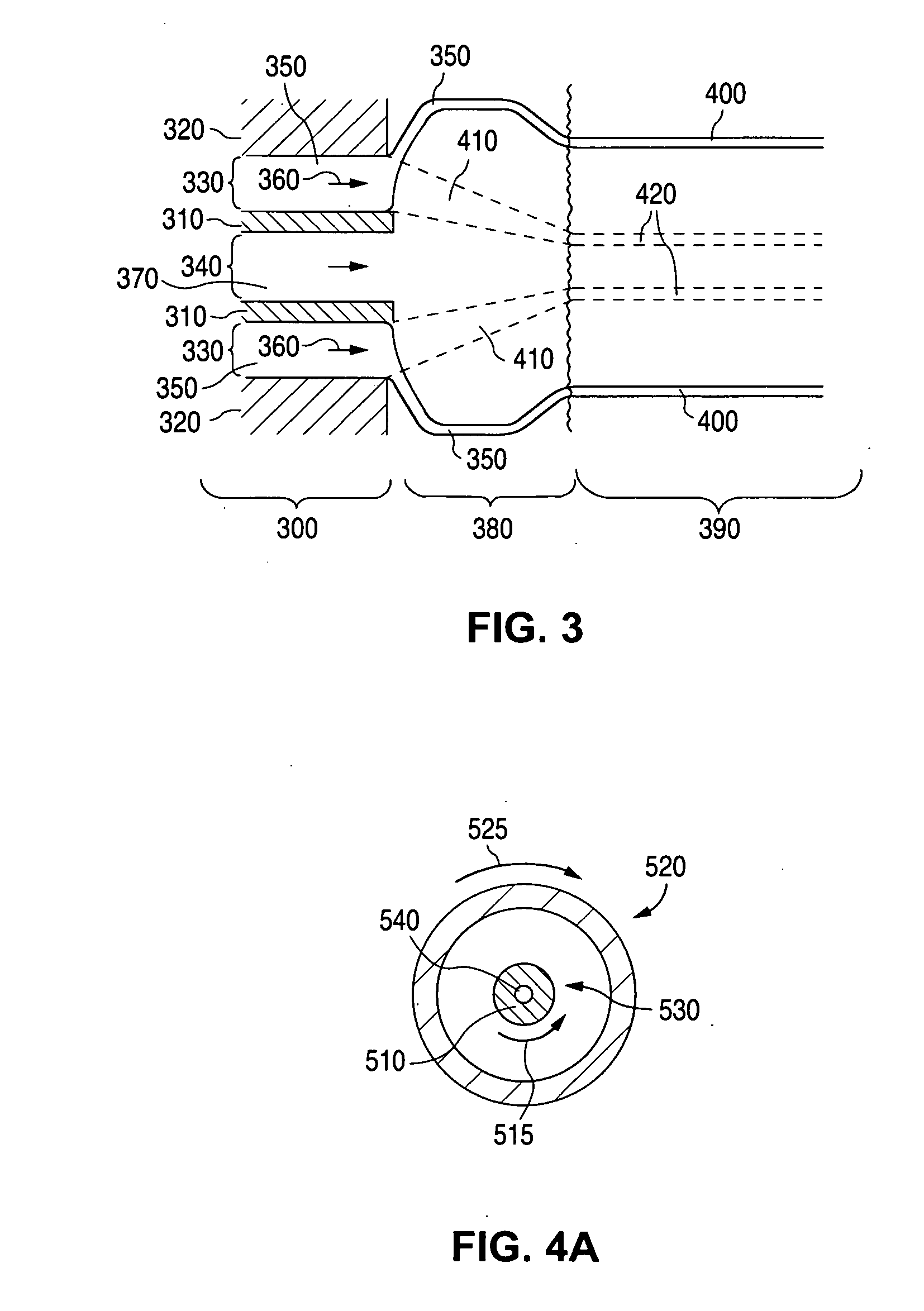
Method of fabricating an implantable medical device with biaxially oriented polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] For the purposes of the present invention, the following terms and definitions apply:
[0026]“Stress” refers to force per unit area, as in the force acting through a small area within a plane. Stress can be divided into components, normal and parallel to the plane, called normal stress and shear stress, respectively. True stress denotes the stress where force and area are measured at the same time. Conventional stress, as applied to tension and compression tests, is force divided by the original gauge length.
[0027] The term “elastic deformation” refers to deformation of an object in which the applied stress is small enough so that the object retains its original dimensions or essentially its original dimensions once the stress is released.
[0028]“Elastic limit” refers to the maximum stress that a material will withstand without permanent deformation.
[0029] The term “plastic deformation” refers to permanent deformation that occurs in a material under stress after elastic limi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com