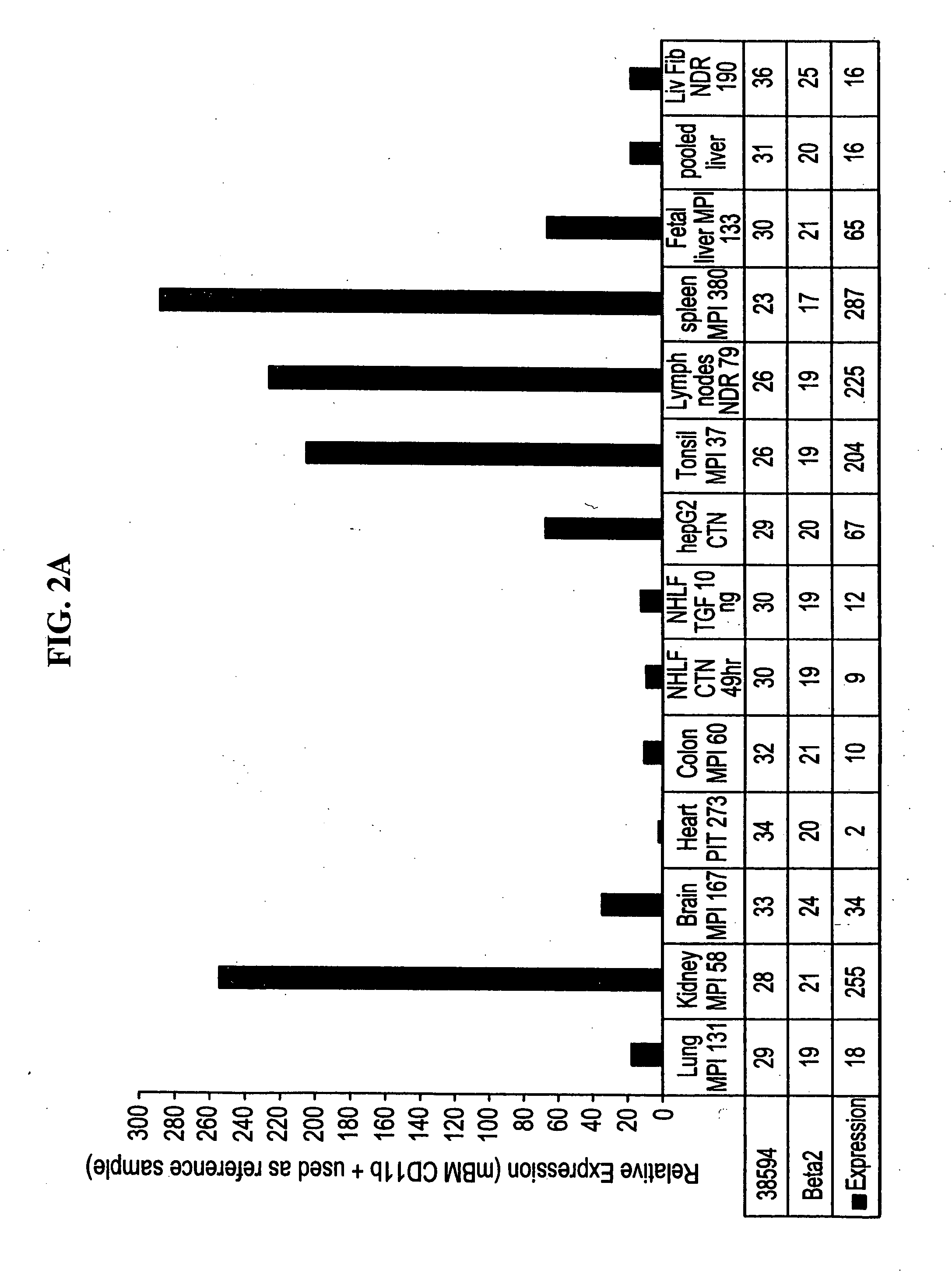
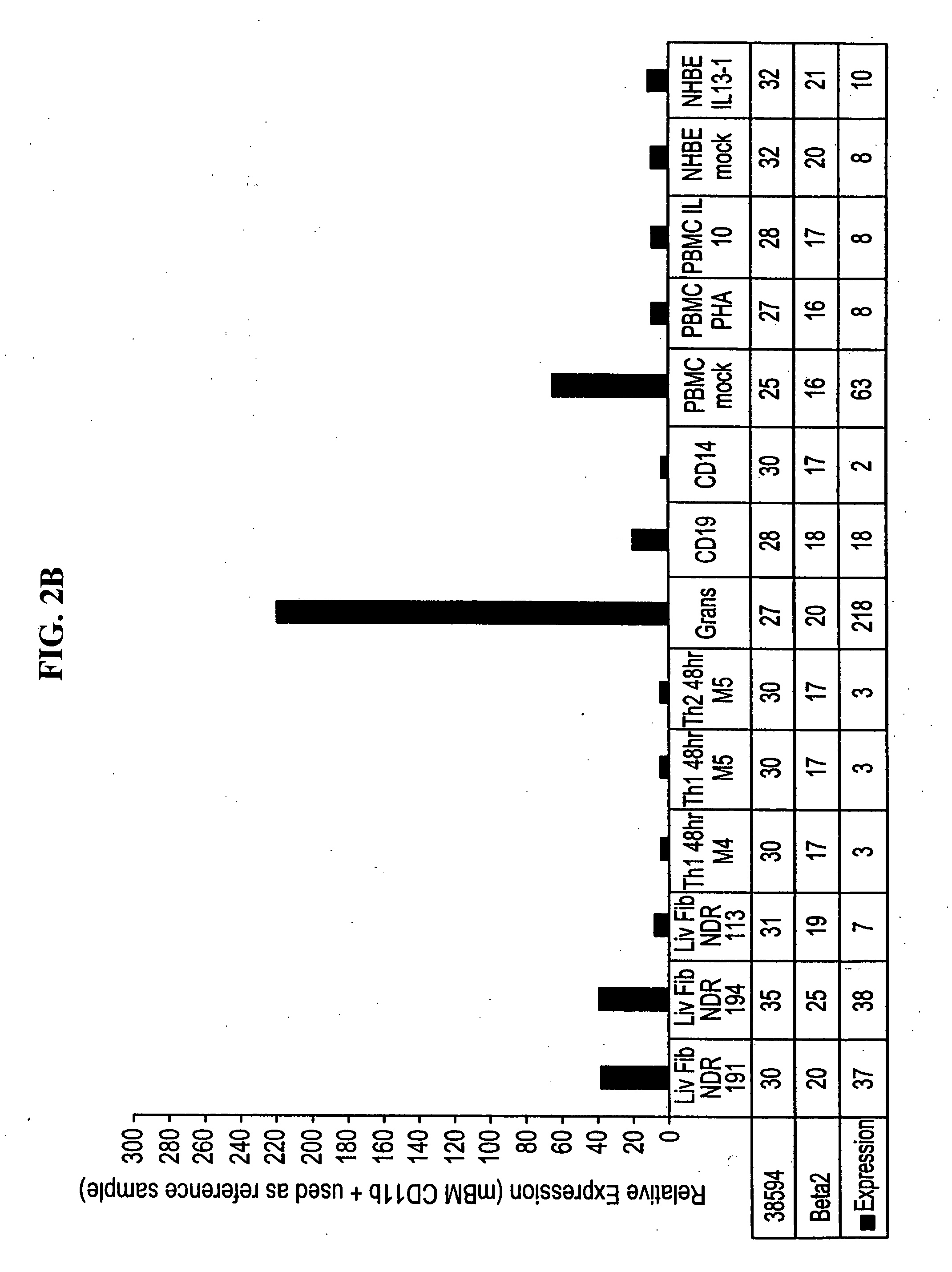
Novel 38594, 57312, 53659, 57250, 63760, 49938, 32146, 57259, 67118, 67067, 62092, FBH58295FL, 57255, and 57255alt molecules and uses therefor
a technology of fbh58295fl and molecule, which is applied in the field of new 38594, 57312, 53659, 57250, 63760, 49938, 32146, 57259, 67118, 67067, etc., can solve problems such as uncontrolled growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification and Characterization of Human MTP-1 cDNA
[1018] In this example, the identification and characterization of the gene encoding human MTP-1 (clone Fbh38594) is described.
Isolation of the MTP-1 cDNA
[1019] The invention is based, at least in part, on the discovery of a human genes encoding a novel protein, referred to herein as MTP-1. The entire sequence of human clones Fbh38594, was determined and found to contain an open reading frame termed human “MTP-1”. The MTP-1 protein sequence set forth in SEQ ID NO:2 comprises about 2144 amino acids. The coding region (open reading frame) of SEQ ID NO:1, is set forth as SEQ ID NO:3.
Analysis of the Human MTP-1 Molecule
[1020] An analysis of the possible cellular localization of the MTP-1 protein based on its amino acid sequence was performed using the methods and algorithms described in Nakai and Kanehisa (1992) Genomics 14:897-911, and at http: / / psort.nibb.ac.jp. The results of the analysis show that human MTP-1 (SEQ ID NO:2...
example 2
Expression of Recombinant MTP-1, OAT, HST-1, TP-2, PLTR-1, TFM-2, TFM-3, 67118, 67067, 62092, HAAT, HST-4 and HST-5 Polypeptides in Bacterial Cells
[1026] In this example, In this example, human MTP-1, human OAT, human HST-1, human TP-2, human PLTR-1, human TFM-2, human TFM-3, human 67118, human 67067, human 62092, human HAAT, human HST-4 and / or human HST-5 is expressed as a recombinant glutathione-S-transferase (GST) fusion polypeptide in E. coli and the fusion polypeptide is isolated and characterized. Specifically, MTP-1, OAT, HST-1, TP-2, PLTR-1, TFM-2, TFM-3, 67118, 67067, 62092, HAAT, HST-4 and / or HST-5 is fused to GST and this fusion polypeptide is expressed in E. coli, e.g., strain PEB199. Expression of the GST- MTP-1, GST-OAT, GST-HST-1, GST-TP-2, GST-PLTR-1, GST-TFM-2, GST-TFM-3, GST-67118, GST-67067, GST-62092, GST-HAAT, GST-HST-4, or GST-HST-5 fusion protein in PEB199 is induced with IPTG. The recombinant fusion polypeptide is purified from crude bacterial lysates of the...
example 3
Expression of Recombinant MTP-1, OAT, HST-1, TP-2, PLTR-1, TFM-2, TFM-3, 67118, 67067, 62092, HAAT, HST-4 and HST-5 Protein in Cos Cells
[1027] To express the MTP-1, OAT, HST-1, TP-2, PLTR-1, TFM-2, TFM-3, 67118, 67067, 62092, HAAT, HST-4, or HST-5 gene in COS cells, the pcDNA / Amp vector by Invitrogen Corporation (San Diego, Calif.) is used. This vector contains an SV40 origin of replication, an ampicillin resistance gene, an E. coli replication origin, a CMV promoter followed by a polylinker region, and an SV40 intron and polyadenylation site. A DNA fragment encoding the entire MTP-1, OAT, HST-1, TP-2, PLTR-1, TFM-2, TFM-3, 67118, 67067, 62092, HAAT, HST-4, or HST-5 protein and an HA tag (Wilson et al. (1984) Cell 37:767) or a FLAG tag fused in-frame to its 3′ end of the fragment is cloned into the polylinker region of the vector, thereby placing the expression of the recombinant protein under the control of the CMV promoter.
[1028] To construct the plasmid, the MTP-1, OAT, HST-1, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| lipophilic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com