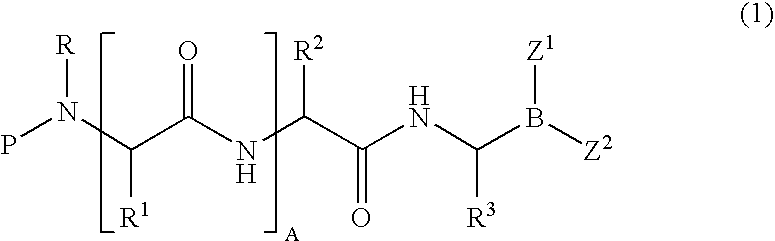
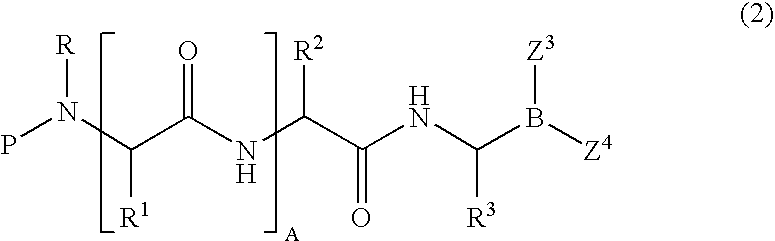
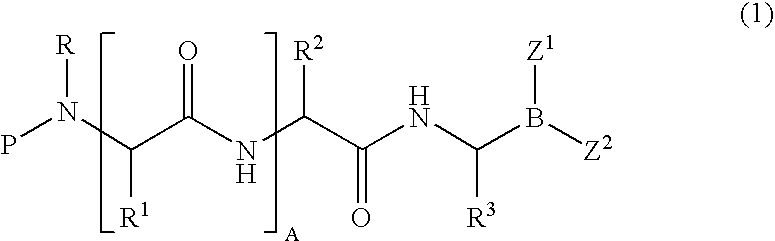
Formulation of boronic acid compounds
a technology of boronic acid and compound, which is applied in the field of pharmaceutical compound formulation, can solve the problems of limited pharmaceutical utility of boronic acid compound, difficult to obtain alkylboronic acid in analytically pure form, and difficult to achieve the effect of enhancing stability, prolonging shelf life and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Lyophilized Formulation of N-(2-pyrazine)-carbonyl-L-phenylalanine-L-leucine Boronic Acid with D-mannitol
[0154] Approximately 40 mg of N-(2-pyrazine)carbonyl-L-phenylalanine-L-leucine boronic acid was weighed into a container, and 16 mL of tert-butanol was added. The container was closed and the suspension was warmed to approximately 45° C. for 5 minutes to complete dissolution of the compound. Water (24 mL) was added with stirring, followed by 0.4 g of mannitol, added as an excipient, 1% w / v. The mixture was stirred to complete dissolution and then cooled to ambient temperature. The solution was filtered through a 0.45 μm nylon membrane. One milliliter aliquots were placed in 5 mL serum bottles. Split rubber stoppers were partially inserted into the bottles, and the bottles were placed in a freeze dryer with a shelf temperature of −45° C. After approximately 1 hour, the vacuum was applied. The shelf temperature was allowed to rise gradually to −35° C. and maintain...
example 2
Production-Scale Preparation of a Lyophilized Formulation of N-(2-pyrazine)carbonyl-L-phenylalanine-L-leucine Boronic Acid with D-mannitol
[0157] In a clean compounding vessel, a solution of 97% tert-butanol / 3% Water for Injection was prepared by warming the required amount of tert-butanol to 35° C. and adding Water for Injection. Approximately 5% of the solution was reserved for use in rinsing. The solution was cooled to 15-30° C., and N-(2-pyrazine)carbonyl-L-phenylalanine-L-leucine boroxine was added with stirring. The container was rinsed with the reserved tert-butanol / water solution, and the rinses were added to the main vessel. The mixture was stirred until the boronic acid compound was completely dissolved. Mannitol was added, with residual mannitol being rinsed into the reaction vessel with fresh Water for Injection. Sufficient Water for Injection was added to reduce the total alcohol content to 40% v / v. The mixture was stirred until the mannitol was completely dissolved. Th...
example 3
Reconstitution of N-(2-pyrazine)carbonyl-L-phenylalanine-L-leucine Boronic Acid
[0165] The lyophilized formulation of N-(2-pyrazine)carbonyl-L-phenylalanine-L-leucine boronic acid with D-mannitol was prepared as described in Example 1. One sample was reconstituted with 2 mL of water. Dissolution was complete within 1-2 minutes of shaking. The entire solution was transferred to a volumetric flask, diluted, and analyzed by HPLC for content of N-(2-pyrazine)carbonyl-L-phenylalanine-L-leucine boronic acid. The total drug content was 1.09 mg. A second sample was reconstituted with 1 mL of propylene glycol:EtOH:H2O, 40:10:50. Dissolution was complete with 1 minute of shaking. The total drug content was 1.11 mg.
[0166] The lyophilized formulation was also reconstituted with 0.9% w / v saline. The lyophilized material dissolved readily at concentrations up to 6 mg / mL. By contrast, solid N-(2-pyrazine)carbonyl-L-phenylalanine-L-leucine boronic acid was not soluble in 0.9% w / v saline at a conce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com