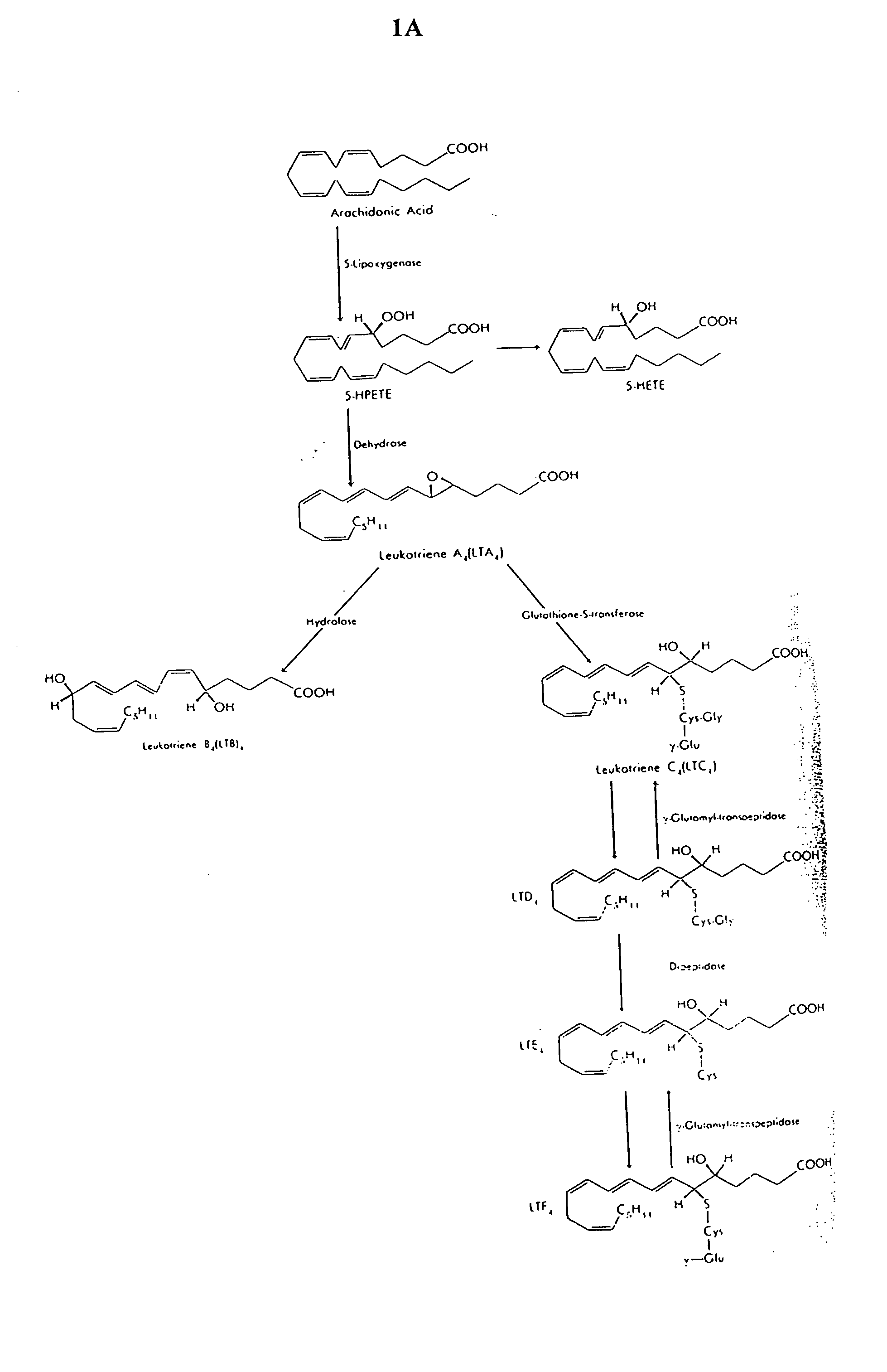
Administration of products of the 5-lipoxygenase metabolic pathway to enhance antimicrobial defense
a technology of lipoxygenase and metabolic pathway, which is applied in the direction of biocide, drug composition, and elcosanoid active ingredients, can solve the problems of increasing difficulty in eradication, affecting the eradication of pneumonia, and compromising bacterial clearance, so as to facilitate bacterial infection eradication, short duration of action, and enhance local endogenous host defense mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Survival Following Intratracheal Klebsiella Challenge in 5-LO Knockout Mice and Wild Type Mice
[0099] Intratracheal instillation of K. pneumoniae in mice is known to cause a reproducible pneumonia characterized by acute pulmonary inflammation that, depending on the inoculum, either resolves or results in death. [G. Rosen et al., FASEB J. 9:200-209 (1995)]. To assess the role of 5-LO products in pulmonary host defense, this example compares the survival of 5-LO knockout and wild type mice.
Profiles of Eicosanoids
[0100]FIGS. 2A and B depict RP-HPLC profiles of radioactive eicosanoids released by prelabeled alveolar macrophages obtained from wild type mice (FIG. 2A) and 5-LO knockout mice (FIG. 2B). The profiles were obtained by prelabeling 106 alveolar macrophages overnight with [3H]arachidonic acid. The alveolar macrophages were then washed and stimulated for 30 minutes with 1 μM A23187. The medium was subjected to lipid extraction and radiolabeled eicosanoids separated by reverse-p...
example 2
Bacterial Clearance Following Intratracheal Klebsiella Challenge in 5-LO Knockout Mice and Wild Type Mice
[0103] As set forth in the preceding example, early events following bacterial challenge (i.e., approximately two-days post-challenge) are important. This example further explores those results by assessing lung homogenate and plasma CFUs at 30 and 48 hours after K. pneumoniae administration.
[0104] Knockout mice and wild type mice were inoculated with 50 CFU intratracheally, and lung homogenate levels and plasma CFU values were determined 48 hours later. FIG. 4 graphically depicts the clearance of K. pneumoniae from lung and plasma after challenge in 5-LO knockout mice (cross-hatched bars) and wild type mice (solid bars) (bars represent mean±SE; n=5-19 animals; *p5), while no wild type mice had bacteria in their plasma at this time point (data not shown).
[0105] These data confirm the importance of an intact leukotriene-generating system for the early containment of a pulmonary...
example 3
Effect of Leukotriene Deficiency and Exogenous Leukotrienes on Alveolar Macrophage Antibacterial Functions In Vitro
[0106] The experiments of this example assess the ability of the alveolar macrophages themselves, the first line of cellular defense, to phagocytose and kill K. pneumoniae in vitro and the effect of administering exogenous leukotrienes on alveolar macrophage antibacterial functions in vitro.
Phagocytic and Bactericidal Activities of Alveolar Macrophages from 5-LO Knockout and Wild Type Mice
[0107] Alveolar macrophages were purified by adherence of bronchoalveolar lavage cells lavaged from uninfected knockout and wild type animals, and preincubated for 5 minutes with 5% K. pneumoniae-specific immune serum (as a source of both complement and specific opsonizing antibody) prior to assays. Cultured alveolar macrophages from either group of mice were incubated in the presence of specific serum with K. pneumoniae for 1 hour and then washed, after which monolayers were either...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com