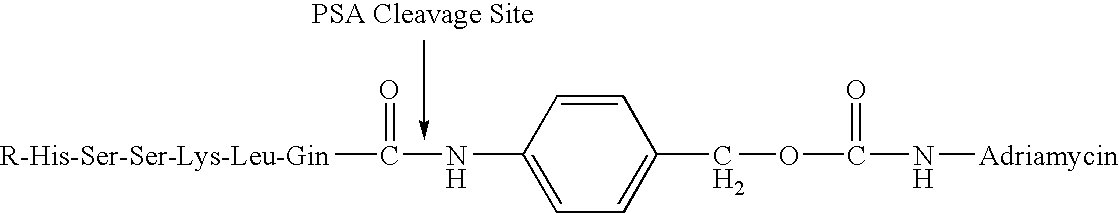
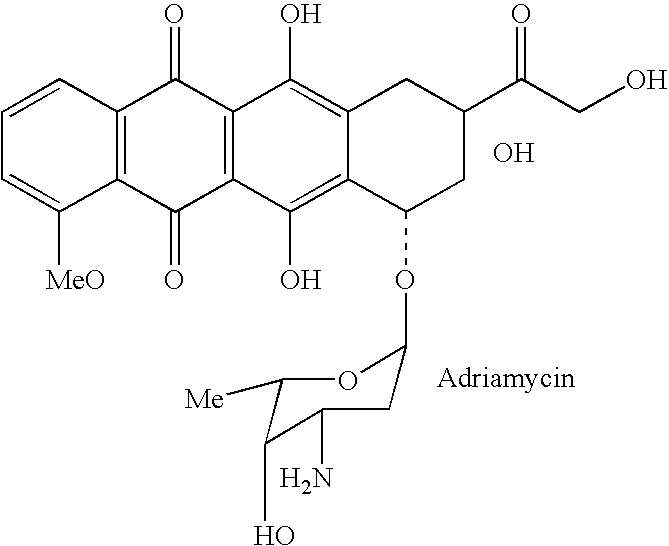
Drug complex for treatment of metastatic prostate cancer
a prostate cancer and drug complex technology, applied in the direction of drug compositions, antibody medical ingredients, peptide/protein ingredients, etc., can solve the problem of limited number of treatments available, and achieve the effect of lowering toxicity and high efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
In Vitro Testing of Drug Complexes
[0034] PC3 and DU-145 androgen-independent human prostate cancer cell lines obtained from the American Tissue Culture Collection (ATCC) are used for in vitro studies. Cells are grown as monolayer cultures in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine and antibiotics. Radiation survival curves and radiation-induced apoptotic DNA fragmentation patterns in these cell lines are already established in the laboratory. Cells are treated with this drug, carrier-peptide, or carrier-peptide-drug complex with or without radiation. After 24 hours, the cells are trypsinized and plated for clonogenic survival studies. Apoptotic response to the treatments is determined by: 1) agarose gel electrophoresis analysis of DNA fragmentation at 48 and 72 hours after treatment, 2) morphological observations after staining with DAPI, and 3) terminal deoxytransferase-mediated dUTP Nick End Labeling (TUNEL) assay.
example 2
In Vivo Testing of Drug Complexes
[0035] Two human prostate carcinoma tumor lines grown as xenografts in male SCID mice are used: the human DU-145 prostate carcinoma which is not androgen dependent and the human LNCaP prostate carcinoma which produces PSA and has a well characterized androgen receptor and response to androgens. The drug complexes are administered up to maximally tolerated doses alone and in conjunction with fractionated radiation therapy (137Cs Gamma Cell 40) delivered to the tumor bearing limb.
[0036] The progress of each tumor is assessed thrice weekly by caliper measurements until the tumors reach 2000 mm3. Tumor growth delay is calculated as the number of days for each tumor to reach a volume of 500 mm3 as compared to untreated controls. The efficacy of combination treatments is assessed using isobologram analysis for determination of additivity / synergy. [0037] PHASE I CLINICAL TRIAL: (MAXIMAL TOLERABLE DOSE ASSESSMENT)
[0038] Based on the results of the in vitr...
example 3
Effect of Thapsigargin (TG) on Cell Proliferation In Vitro
[0039] Preliminary in vitro data indicate that TG inhibits cell proliferation (Table 1, DU 145 cells) and clonogenic cell survival (Table 2, PC3 cells) of androgen independent human prostate cancer cells.
[0040] Table 1 shows the effect of thapsigargin and radiotherapy on proliferation of DU-145 prostate cancer cells. The results indicate that thapsigargin inhibits cell proliferation vitro.
TABLE 1Effect of TG and RT on proliferation of DU-145 prostate cancer cells*ThapsigarginTotal Number of Cells per Dish (106)(nM)0 Gy2 Gy4 Gy8 Gy02.11.41.20.5201.00.80.30.41000.70.50.50.44000.70.40.30.3
*Cells were irradiated 2 hours after adding TG or DMSO. At 48 hours cells were trypsinized and counted after staining with trypan blue. <5% of the cells were trypan blue + at that time.
[0041] Table 2 shows the effect of thapsigargin and radiotherapy on clonogenic cell survival of PC3 prostate cancer cells. The results indicate that thapsig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com