Method of treating anemia caused by ribavirin treatment of hepatitis C using erythropoietin alpha
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
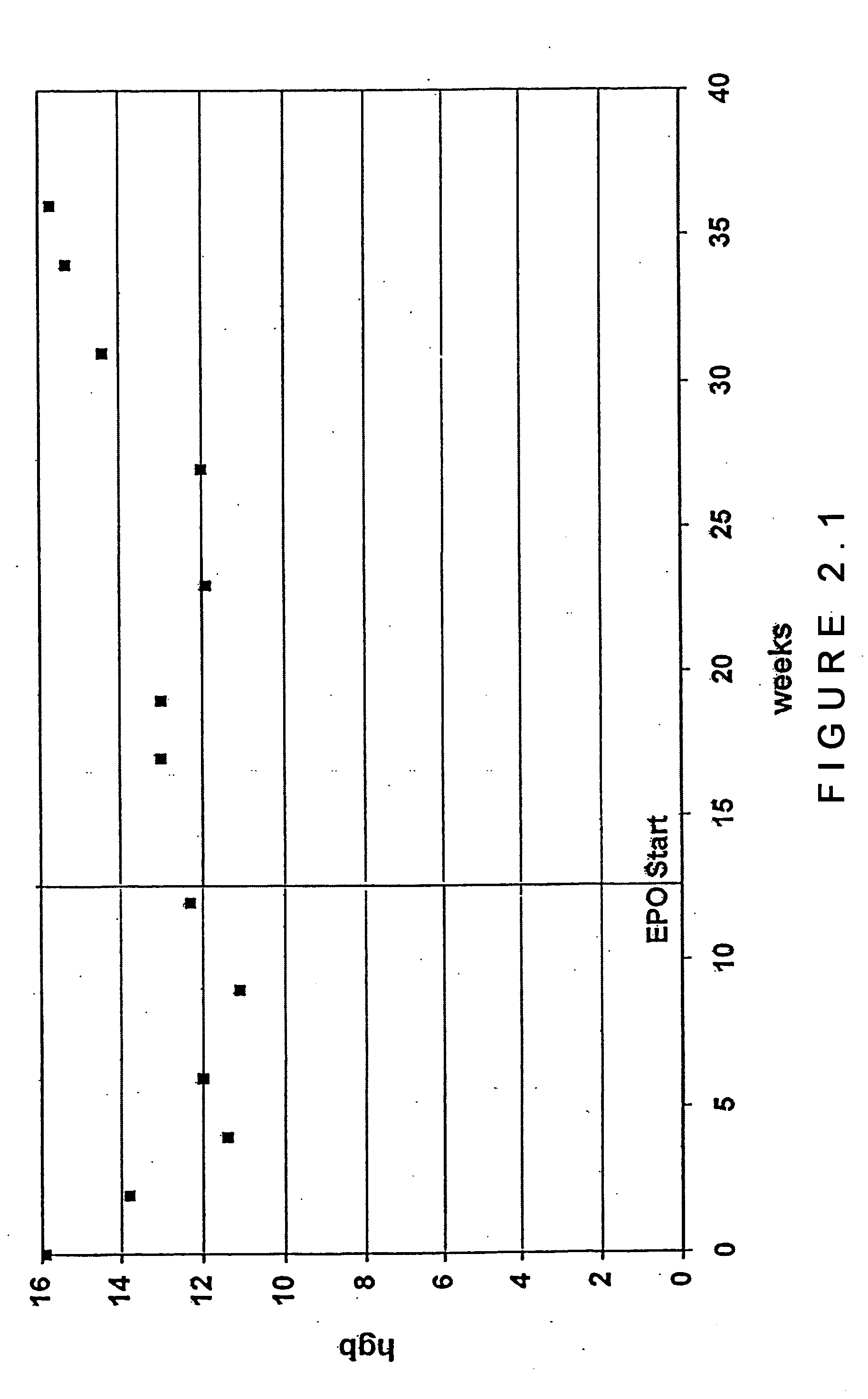
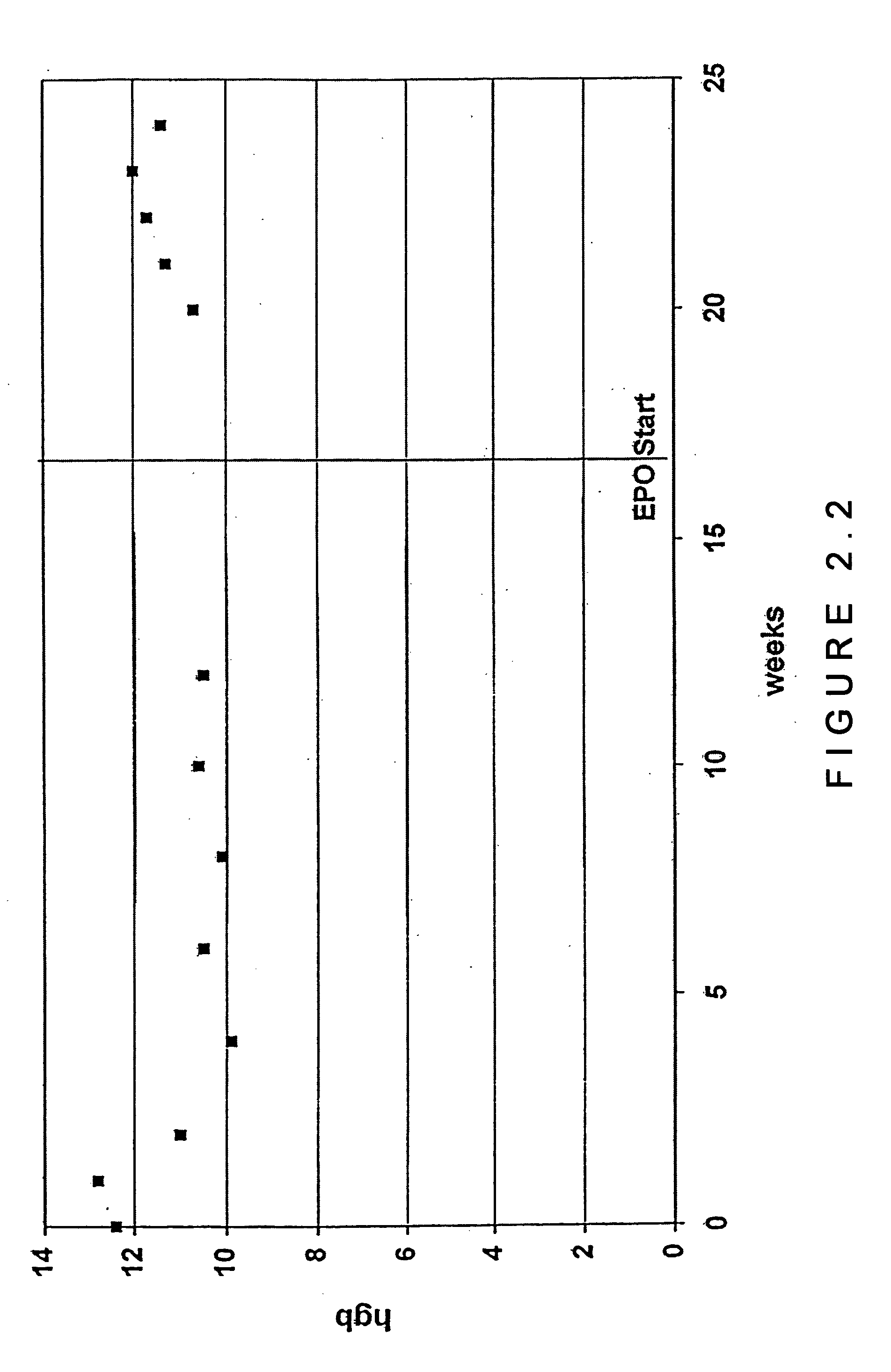
[0059] In an open-label, prospective pilot study, 56 subjects with chronic HCV infection were treated with both RBV and IFN-α. Eighteen subjects with anemia (hemoglobin 2 g / dl) and reduced exercise tolerance following IFN-α / RBV initiation were treated with Epoetin alpha (up to 40,000 units per week subcutaneously). Remaining nonanemic subjects (38 / 56) who did not receive Epoetin alpha served as a control group. The two groups were followed for means of 25.3 and 21.2 weeks, respectively. At baseline, mean hemoglobin levels were not significantly different in the subjects who received epoetin alpha compared to those who did not receive the medication (14.3 g / dl vs. 15.1 g / dL, n.s.). At the time of epoetin alpha initiation, the mean hemoglobin level declined 25.4% (to 10.6 g / dL) in the group receiving this treatment. At the last study visit, mean hemoglobin levels were 12.7 g / dL among patients receiving epoetin alpha vs. 13.0 g / dL among controls (difference n.s.) reflecting a recovery ...
example 2
[0060] A study of interferon alone and interferon plus ribavirin for hepatitis C in HIV-infected patients was performed. Ten patients received interferon alone and 11 received the combination treatment of interferon alpha and ribavirin. Median liver biopsy fibrosis score for both groups was 2.1. In the interferon alone group, at 3 months, there was no change in HCV RNA, CD4 dropped from a median of 190 to 186 cells / ml and HIV RNA dropped from a median of 1500 copies per ml to less than 400. At the same time point, 3 months, in the combination group, the HCV RNA dropped from 3.2 million to less than about 600 copies / ml. CD4 cells dropped from a median of 544 to 237, but percentage CD4 cells did not change. The main adverse event was anemia which occurred in 5 / 21(23.8%). This was treated with erythropoietin 40,000 units weekly when the hemoglobin declined to to 10 g / dl. This treatment resulted in a rise in hemoglobin to 12.7 g / dl after a median of 4 weeks treatment. This was the first...
example 3
[0062] Fifty-six chronically HCV-infected (>90 days) subjects who were treated with IFN-α (3 million units TIW) and RBV (1000 mg / d for subjects 75 kg) were entered into an open-label, pilot, prospective study at several centers. Subjects were excluded if they were infected with the human immunodeficiency virus type-i or had a history of anemia, chronic renal disease, or coronary artery disease. RBV doses were adjusted by treating physicians as clinically indicated throughout the study.
[0063] Following INF-α / RBV initiation, subjects with new-onset anemia (hemoglobin 2 g / dL decrease in hemoglobin) or a decrease in hemoglobin accompanied by decreased exercise tolerance (providers' impressions) received epoetin alpha (up to 40,000 units per week, subcutaneously). Subjects who did not become anemic and who did not receive epoetin alpha served as a control group.
[0064] After initiation of epoetin alpha therapy, subjects were evaluated at a 2-week interval for 8 weeks and every 4 weeks t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com