Compositions and methods for eliminating undesired subpopulations of T cells in patients with immunological defects related to autoimmunity and organ or hematopoietic stem cell transplantation
a technology for autoimmunity and hematopoietic stem cells, applied in the field of compositions and methods for eliminating undesired subpopulations of t cells in patients with immunological defects, can solve the problems of inability to regenerate the supply of accessory cells, cells may contaminate the entire t cell population during long-term culture, and the increased robustness and ease of t cell preparation remain less than ideal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Deletion of Antigen-Specific T Cells Following Restimulation with CD3 / CD28 XCELLERATE™ Beads
[0208] This example describes the elimination of antigen-specific T cells from a mixed population of cells by restimulation with anti CD3 / CD28 XCELLERATE™ beads (3×28 beads). The generation of XCELLERATED T cells™ using the processes described herein is essentially as described in U.S. patent application Ser. No. 10 / 133,236.
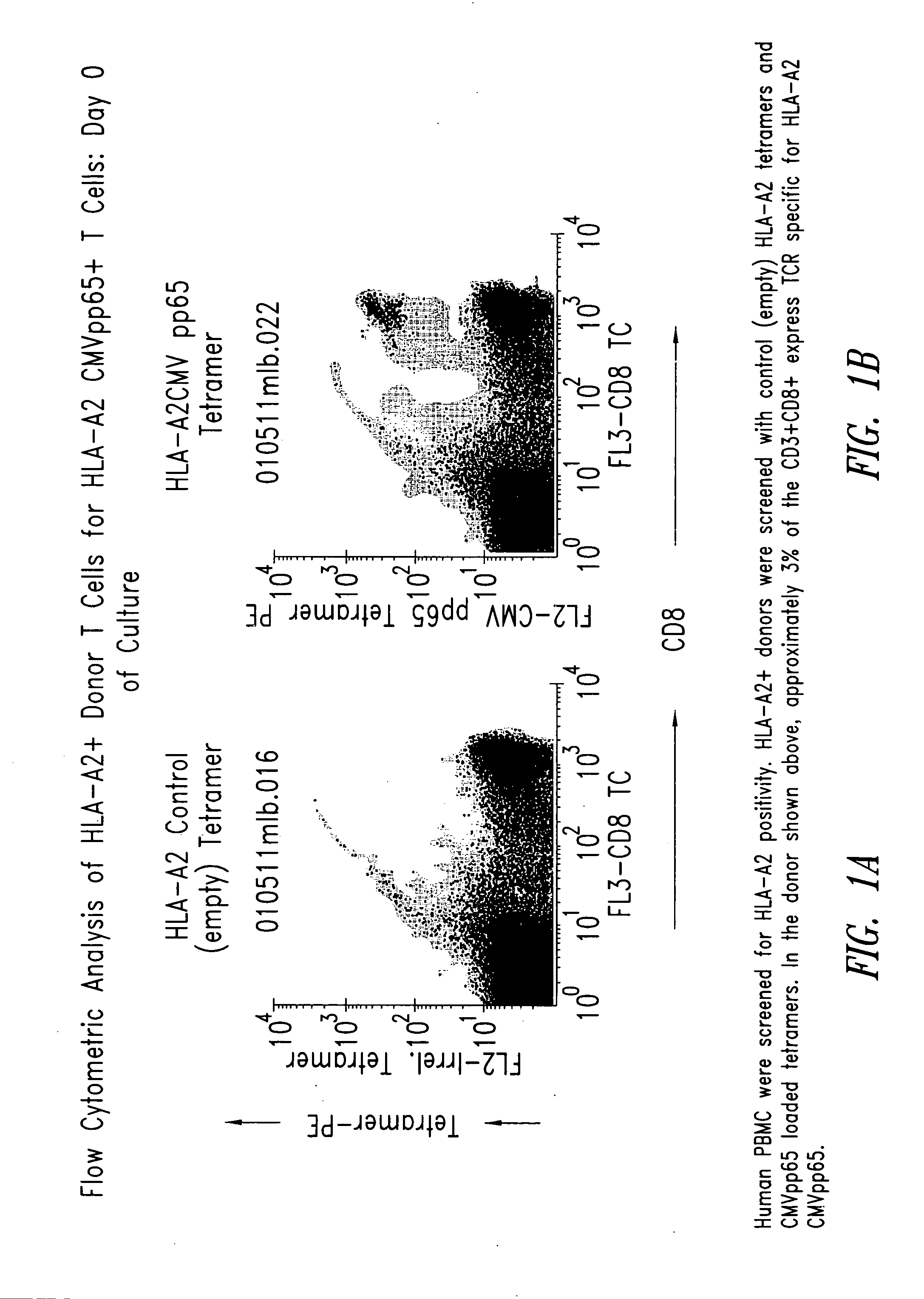
[0209] Human PBMC were screened for HLA-A2 CMVpp65 positivity by flow cytometry using HLA-A2 tetramers loaded with CMVpp65 peptide (HLA-A2-CMVpp65). Approximately 3% of the CD3+ CD8+T cells in the donor selected expressed TCR specific for HLA-A2-CMVpp65 (FIG. 1).
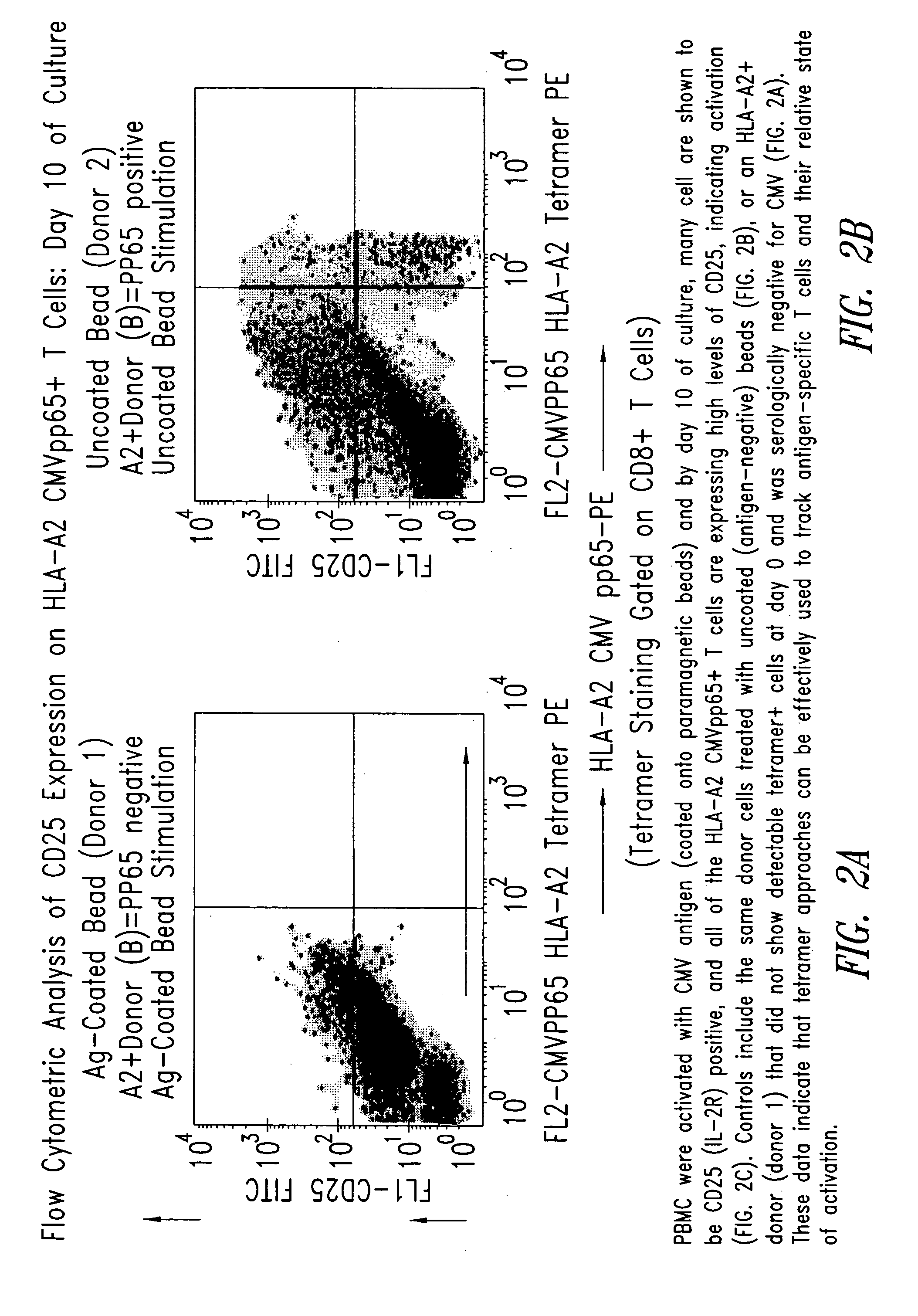
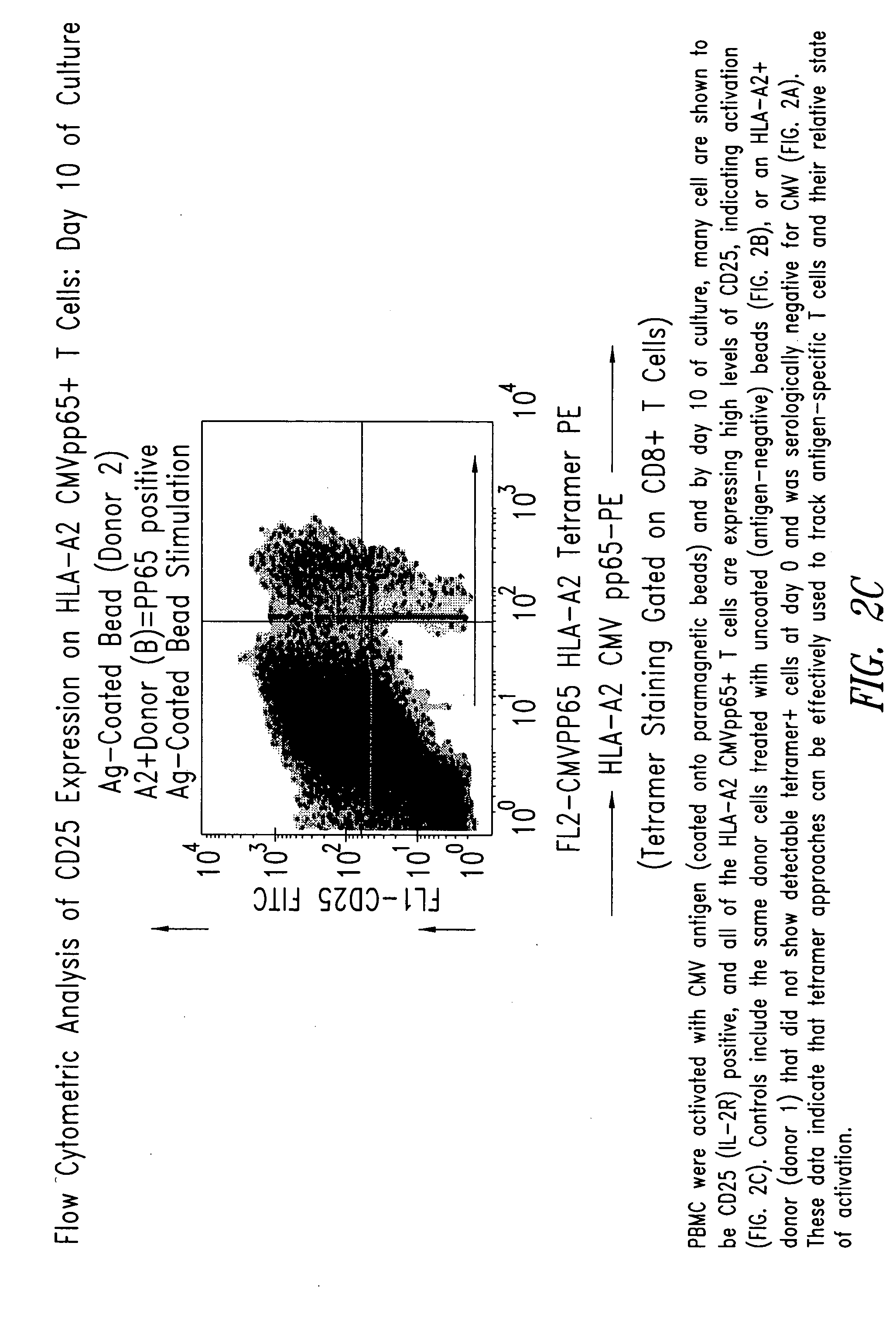
[0210] PBMC from the donor (donor 2) and control donor (donor 1) were activated with CMV antigen coated onto paramagnetic beads and by day 10 of culture, many cells were shown by flow cytometric analysis to be CD25 (IL-2R) positive, and all of the HLA-A2 CMVpp65+T cells expressed high levels of CD25, indicating ...
example 2
Determination of Apoptosis
[0213] This example describes an illustrative assay for measuring apoptosis.
[0214] DNA Fragmentation Assay: Cells are lysed in 50 μl of lysis buffer (10 mM EDTA, 50 mM Tris pH 8, 0.5% sodium dodecyl sulfate, 0.5 mg / ml proteinase K). RNAse A (0.5 mg / ml) is added and lysates are incubated for 1 hr. at 37° C. Two phenol extraction (equal volumes) are performed, followed by one chloroform extraction. DNA is precipitated with two volumes of ice-cold ethanol and incubated at −80° C. for 1 hr. DNA is pelleted by centrifugation at 14,000 rpm for 10 minutes at 4° C. Pellets are air-dried for 30 minutes, resuspended in 50 μl of Tris-EDTA pH 8. DNA is electrophoresed in a 1.8% agarose gel in 1×TBE running buffer (0.05 M Tris base, 0.05 M boric acid, 1 mM disodium EDTA), according to the methods of Preston, et al., Cancer Res., 1994, 54, 4214-4223.
example 3
Induction of Apoptosis in B-Cells by Coculture with XCELLERATED T Cells™
[0215] This example describes the deletion of leukemic B-cells in B-CLL patient samples by co-culture with XCELLERATED T cells™.
[0216] XCELLERATED T cells™, generated essentially as described in U.S. patent application Ser. No. 10 / 133,236, were co-cultured with unmanipulated autologous leukemic cells from B-CLL patients. Cell surface markers for CD54, CD80, CD95 (FAS) and CD86, and Annexin / PI (apoptosis) were measured at 24 and 48 hours by flow cytometry. XCELLERATED T cells™ were shown to drive up expression of CD95 (FAS) on leukemic B cells (FIG. 4). After 48 hours of co-culture with day 12 XCELLERATED T cells™, autologous leukemic B cells show increased expression of CD95 and sensitivity to anti-FAS as measured by flow cytometry (FIG. 5). As shown in FIG. 5, addition of anti-FAS antibody to co-cultured T:B cells led to increased apoptosis in the leukemic B-cells. In an additional study, it was shown that T c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time period | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
| incubation time period | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com