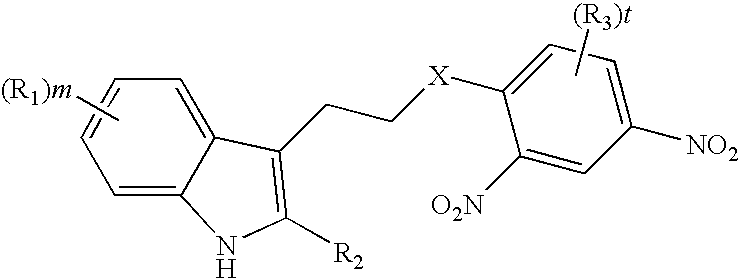
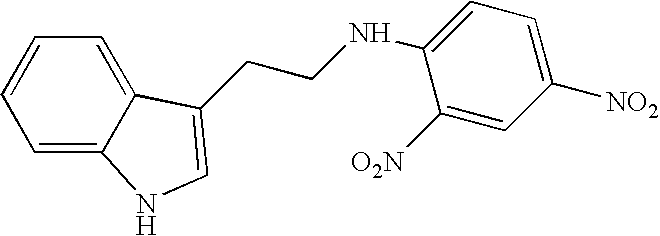
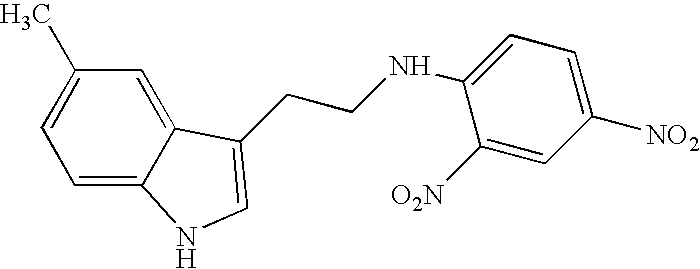
Derivatives of tryptamine and analogous compounds, and pharmaceutical formulations containing them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-(2,4-dinitrophenyl)tryptamine (ML-25)
1 mMole of tryptamine was dissolved in 100 ml of water and the pH was adjusted to pH 8.3 with 2.5 moles of sodium bicarbonate (NaHCO3), A 1.5% solution of 2,4-dinitrofluorobenzene in 200 liters ethanol was added and the mixture was stirred during 2 hours at room temperature. The desired product precipitates out, it is washed and dried. The product is obtained in 90% yield, and TLC (chloroform, silica-gel plates, reveals one yellow spot (Rf=0.84) which is well resolved from the starting materials under the same conditions.
example 2
N-(2,4-dinitrophenyl)-5-methyltryptamine (ML-28)
1 mMole of 5-metyltryptamine was dissolved in 100 ml of water and the pH was adjusted to pH 8.3 with 2.5 moles of sodium bicarbonate (NaHCO3), A 1.5% solution of 2,4-dinitrofluorobenzene in 200 ml ethanol was added and the mixture was stirred during 2 hours at room temperature. The desired product precipitates out, it is washed and dried. The product is obtained in 85% yield, and TLC (chloroform, silica-gel plates, reveals one yellow spot (Rf=0.8) which is well resolved from the starting materials under the same conditions.
example 3
2,4-dinitro-5-tryptylaminoacetanilide (ML-26)
1 mMole of tryptamine was dissolved in 100 ml of water and the pH was adjusted to pH 8.3 with 2.5 moles of sodium bicarbonate (NaHCO3), A 1.5% solution of 2,4-dinitro-5-fluoroacetanilide in 200 ml ethanol was added and the mixture was stirred during 2 hours at room temperature. The desired product precipitates out, it is washed and dried. The product is obtained in 80% yield, and TLC (chloroform, silica-gel plates, reveals one yellow spot (Rf=0.76) which is well resolved from the starting materials under the same conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com